Which statement accurately describes the energy needs for photosynthesis and cellular respiration?
Solar energy is needed for cellular respiration but not for photosynthesis.
Chemical energy in the form of glucose is needed for photosynthesis, and solar energy is needed for cellular respiration
Chemical energy in the form of glucose is needed for photosynthesis, and salar energy is needed for cellular respiration.
Solar energy is needed for photosynthesis and chemical energy in the form of glucose is needed for cellular respiration
Answers
Answer:
Solar energy is needed for photosynthesis and chemical energy in the form of glucose is needed for cellular respiration
Explanation:
The energy needs for photosynthesis and cellular respiration involves the use of solar energy for photosynthesis and chemical energy for cellular respiration.
Solar energy is the energy derived from the sun. This form of energy is very essential to the process of photosynthesis. During photosynthesis, green plants manufacture their food in the presence of carbon dioxide and water using sunlight.
At cellular respiration, organisms derive energy by the conversion of chemical energy from plants into other forms of useful energy. The chemical energy from the sun is usually in the form of glucose.
Answer: Solar energy is needed for photosynthesis and chemical energy in the form of glucose is needed for cellular respiration
Explanation:
Related Questions
If the sun rises at 7:36am and sets at 6:00pm, what times would we use for our data in the 24-hour clock format?
A.
sunrise: 0736
sunset: 1600
B.
sunrise: 0736
sunset: 0600
C.
sunrise: 0736
sunset: 1800
D.
sunrise: 1936
sunset: 0600
E.
None of the above.
Answers
How should the magnetic field lines be drawn for the magnets shown below?
Answers
Answer:
Magnetic field lines can be drawn by moving a small compass from point to point around a magnet. At each point, draw a short line in the direction of the compass needle. ... This is shown in Figure 20.11, which shows the magnetic field lines created by the two closely separated north poles of a bar magnet.
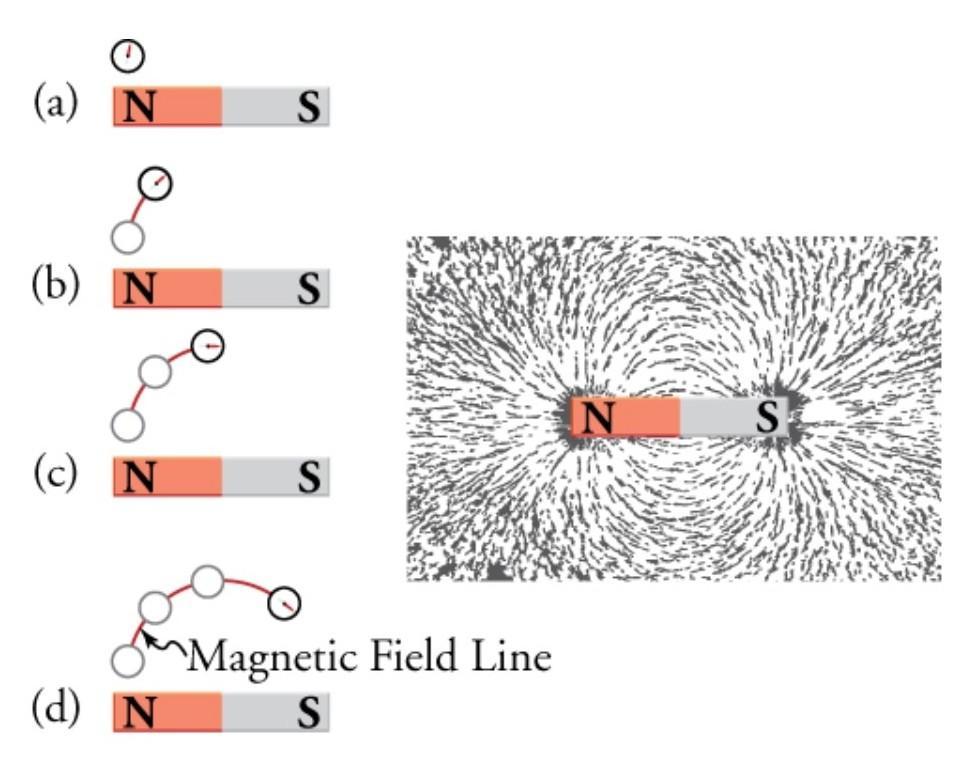
The direction of magnetic field lines is north to south. Therefore, option A is correct.
What are magnetic field lines?The magnetic field surrounding a magnet or current-carrying wire is represented visually by magnetic field lines. They are used to show the strength and direction of the magnetic field at various locations across space.
The magnetic field of a magnet constantly flows from its north pole to its south pole, forming closed loops along the field lines. With closely spaced field lines suggesting a greater magnetic field and widely spread field lines indicating a lesser magnetic field, the density of the magnetic field lines determines the strength of the magnetic field.
The direction of the magnetic field lines can be determined using a compass. Thus. option A is correct.
Learn more about magnetic field lines, here:
https://brainly.com/question/19646982
#SPJ7
Your question is incomplete, most probably the full question is this:
How should the magnetic field lines be drawn for the magnets shown below?
Write the symbol for every chemical element that has atomic number greater than 55 and less than 140.8 u
Answers

Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as the solvent and the other has carbon tetrachloride as the solvent. Determine that molal concentration, m (or b), and boiling point, Tb.
Given
Ethanol
normal Boiling point: 78.4
Kb: 1.22
CCL4
normal boiling point: 76.8
Kb: 5.03
Answers
Answer: m = 0.42; Tb = 79°
Explanation: The relationship between boiling point of the solvent above a solution is directly proportional to the molal concentration of the solute, i.e.:
ΔT = \(K_{b}.m\)
where
ΔT is the change in boiling point of the solvent;
\(K_{b}\) is the molal boiling point elevation constant;
m is the molal concentration of the solute in the solution;
For there two solutions:
1) Ethanol:
ΔT = \(K_{b}.m\)
Tb - \(T_{normal} = K_{b}.m\)
Tb - 78.4 = 1.22.m (1)
2) Carbon Tetrachloride:
Tb - \(T_{normal} = K_{b}.m\)
Tb - 76.8 = 5.03.m (2)
Solving the system of equations:
Tb - 78.4 = 1.22.m
Tb = 1.22.m + 78.4 (3)
Substituing (3) in (2)
1.22.m + 78.4 - 76.8 = 5.03m
3.81m = 1.6
m = 0.42
With m, find Tb:
T - 76.8 = 5.03.0.42
T = 2.11 + 76.8
T = 79°
Molal concentration is 0.42 and boiling point is 79°
Consider a three-dimensional model of methane as seen here in two dimensions. If we use VSEPR theory to describe the geometry of a methane molecule we say its shape is. A) Linear. B) Tetrahedral. C)Trigonal Planar. D)Trigonal Bipyramidal
Answers
Answer:B
Explanation:
Tetrahedral I took the test
Answer:
B is the answer'
Explanation:
2. Which chemical below is easier to dissolve in water
a) KBr b) CO2 c)CH4
d) O2
Answers
The correct answer is a) KBr.
KBr is an ionic compound composed of a metal (K) and a non-metal (Br). When this compound is added to water, the polar water molecules surround the ions in the solid and separate them, which leads to the compound dissolving in water.
What is Ionic Compound?
An ionic compound is a chemical compound composed of ions held together by electrostatic forces called ionic bonds. Ions are atoms or molecules that have gained or lost one or more electrons, giving them a positive or negative charge. In an ionic compound, a positively charged ion (cation) and a negatively charged ion (anion) are attracted to each other to form a stable compound.
CO2, CH4, and O2 are nonpolar molecules, and therefore, do not dissolve well in water. CO2 and O2 are gases at room temperature and pressure, while CH4 is a gas at room temperature but can be liquefied under pressure.
Learn more about Ionic Compound from the given link
https://brainly.com/question/2687188
#SPJ1
The Haber Process is the main industrial procedure to produce ammonia. The reaction combines nitrogen from air with hydrogen mainly from natural gas (methane) and is reversible and exothermic. The enthalpy change for this reaction is - 92 kJ mol-1. In an experiment, 1.5 moles of N2 and 4.0 moles of H2 is mixed in a 1.50 dm3 reaction vessel at 450 °C. After reaching equilibrium, the mixture contained 0.9 mole of NH3.
A) With the above information, write the reaction equilibrium equation in the Haber process. t.
B) Calculate Kc for this reaction.
C) What is the equilibrium yield of ammonia in this reaction?
D) Referring to Le Chatelier's principle and above information, suggest two ways to increase the yield of ammonia in this reaction and explain.
Answers
Answer:
A) \(N_2(g)+3H_2(g)\rightleftharpoons 2NH_3(g)\).
B) \(Kc=0.0933\).
C) 0.9 mol.
D) Increasing both temperature and pressure.
Explanation:
Hello,
In this case, given the information, we proceed as follows:
A)
\(N_2(g)+3H_2(g)\rightleftharpoons 2NH_3(g)\)
B) For the calculation of Kc, we rate the equilibrium expression:
\(Kc=\frac{[NH_3]^2}{[N_2][H_2]^3}\)
Next, since at equilibrium the concentration of ammonia is 0.6 M (0.9 mol in 1.5 dm³ or L), in terms of the reaction extent \(x\), we have:
\([NH_3]=0.6M=2*x\)
\(x=\frac{0.6M}{2}=0.3M\)
Next, the concentrations of nitrogen and hydrogen at equilibrium are:
\([N_2]=\frac{1.5mol}{1.5L}-x=1M-0.3M=0.7M\)
\([H_2]=\frac{4mol}{1.5L}-3*x=2.67M-0.9M=1.77M\)
Therefore, the equilibrium constant is:
\(Kc=\frac{(0.6M)^2}{(0.7M)*(1.77M)^3}\\ \\Kc=0.0933\)
C) In this case, the equilibrium yield of ammonia is clearly 0.9 mol since is the yielded amount once equilibrium is established.
D) Here, since the reaction is endothermic (positive enthalpy change), one way to increase the yield of ammonia is increasing the temperature since heat is reactant for endothermic reactions. Moreover, since this reaction has less moles at the products, another way to increase the yield is increasing the pressure since when pressure is increased the side with fewer moles is favored.
Best regards.
Check
Match each power of a power expression with its simplified expression.
(4-3)-3
(40)-9
(46)-3
(-49)2
ТТІ
49
(-4)18
1
40
Answers
The simplified expression for the given expression is:40T^2 × T^98 × (4^181) / 4^67
Given expression : (4^-3)^-3 × (4^0)^-9 × (4^6)^-3 × (-4)^-49 × (2T)^2 × (T^2)^49 × (-4)^181 × 40To simplify the given expression, we use the following properties of exponents : For any real numbers a, b and n, we have ;a^-n = 1/a^n and a^n × a^m = a^(n+m)Let's simplify each term of the given expression one by one:(4^-3)^-3 = 4^(9) because when a negative exponent is raised to another negative exponent, it becomes positive. (4^-3)^-3 = 4^(-3×-3) = 4^(9)(4^0)^-9 = 4^0 = 1 because any number raised to the power of 0 is equal to 1(4^6)^-3 = 4^(-6×3) = 4^(-18) because when a negative exponent is multiplied by another negative exponent, it becomes positive.(-4)^-49 = -1/(4^49) because when a negative exponent is raised to another negative exponent, it becomes positive and also negative.(-4)^181 = (4^181) because when an odd negative power of a negative number is raised to another power, it becomes negative.40 = 40 as it is(2T)^2 = 4T^2(T^2)^49 = T^(2×49) = T^98(-49) = -49 as it is Now let's simplify the given expression:1 × 1/(4^49) × 4^(-18) × 40 × 4T^2 × T^98 × (4^181)40 and 4^-18 can be simplified and combined as follows:1/(4^49) × 4^(-18) × 40 = 40/(4^49 × 4^18) = 40/4^(49+18) = 40/4^67.
for such more questions on expression
https://brainly.com/question/31591125
#SPJ8
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Consider the reaction: 2Al(s) + Fe_{2}O_{3}(s) → 2Fe(s) + Al_{2}O_{3}(s), ΔH_{rxn} = -851.5 kJ/mol. If 75 g of aluminum are reacted with excess iron(III) oxide, how much heat (in kilojoules) is produced?
Answers
According to the question -638.625 kJ heat is produced.
What is heat?Heat is the transfer of energy from one object or system to another object or system due to a difference in temperature. It is a form of energy that can be transferred from one object to another, or from one region of space to another. Heat is the result of the movement of particles within an object that contain energy.
To solve this problem, we need to use the given reaction and the information about the mass of aluminum. Since the reaction given is a balanced equation, we can use the stoichiometric coefficients from the equation to convert between masses and moles of reactants and products.
First, we can calculate the moles of aluminum needed to react with 75 g of aluminum:
Moles of Al = 75 g Al/ (26.98 g/mol Al) = 2.775 moles Al
Next, using the balanced equation, we can calculate the moles of heat produced by this reaction:
Moles of heat = (2.775 moles Al)(-851.5 kJ/mol) = -2375.88 kJ
Finally, we can convert the moles of heat to kilojoules:
Heat produced (kJ) = (-2375.88 kJ)(1000 J/1 kJ) = -638.625 kJ
To learn more about heat
https://brainly.com/question/25603269
#SPJ1
Consider the following intermediate chemical equations.
2 equations: first: upper C (s) plus one-half upper O subscript 2 (g) right arrow upper C upper O (g). Second: upper C upper O (g) plus one-half upper O subscript 2 (g) right arrow upper C upper O subscript 2 (g).
When you form the final chemical equation, what should you do with CO?
Answers
The CO gas produced in the first equation is used in the second equation to produce CO2 in the final equation.
In the intermediate equations, solid carbon (C) and molecular oxygen (O2) are transformed into gaseous carbon monoxide (CO), which is then reacted with more oxygen to produce carbon dioxide (CO2).
The final chemical equation can be created by combining the intermediate equations and cancelling out the intermediate reactant and product (CO and O2) to obtain the overall balanced equation for the reaction:
C(g) + O2(s) = CO2 (g)
Thus, the CO generated in the first equation is consumed in the second equation and does not show up in the third and final equation. The two intermediate reactions' combined outcome is represented by the final equation, which only includes the reactants (C and O2) and product (CO2).
Learn more about molecular oxygen here:
https://brainly.com/question/11587330
#SPJ1
!Please help! Will give brainliest to correct answer!!
A student collected the data shown in the table below during an experiment.
Liquid Characteristics
Liquid Characteristics
Boiling Point Freezing Point Color Rate of Evaporation
Mercury 357 °C −39 °C shiny silver gray negligible
Alcohol 78 °C −115 °C colorless rapid
Based on the data, which of the following conclusions can be made about the use of mercury and alcohol thermometers?
An alcohol thermometer can measure the freezing point of a liquid that freezes at −80 °C.
An alcohol thermometer can measure a wider range of temperatures in a laboratory.
An alcohol thermometer is more reliable to measure the temperature of a liquid in a beaker that is 80 °C.
An alcohol thermometer is better to measure the boiling points of colorless liquids.
Answers
Answer:
its D
Explanation:
I took the test its right
The data can lead us to conclude that an alcohol thermometer can measure the freezing point of a liquid that freezes at −80 °C..
Liquid in glass thermometersThe liquid in glass thermometers are those thermometers whose thermometric substance is a liquid. Usually the best liquids to be used are those that have a long liquid range.
The data can lead us to conclude that an alcohol thermometer can measure the freezing point of a liquid that freezes at −80 °C..
Learn more about thermometers: https://brainly.com/question/365923
lphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
22.55 mL of an H2SO4 solution
were titrated with 14.85 mL of a
0.146 M NaOH solution to reach the
equivalence point. What is the
molarity of the H2SO4 solution?

Answers
The concentration of H₂SO₄ solution is equal to 0.0480 M.
What is a neutralization reaction?A neutralization reaction is described as a chemical reaction where acid and base react to produce respective salt and water. When a strong acid reacts with a strong base then the salt can be neutral.
When H₂SO₄ (a strong acid) reacts with NaOH, the resulting salt is Na₂SO₃ and water.
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2H₂O
Given, the concentration of NaOH = 0.146 M
The volume of the NaOH = 14.85 ml = 0.01485 L
The number of moles of NaOH, n = M × V = 0.146 × 0.01485 = 0.00216 M
The volume of the H₂SO₄ = 22.55 ml = 0.02255 L
The number of moles of H₂SO₄, n = 0.00216/2 = 0.00108 mol
The concentration of H₂SO₄ =0.00108/0.02255 = 0.0480 M
Therefore, the molarity of H₂SO₄ is 0.0480 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
Question 10 of 35
The graph shows the change in temperature of a sample
of water in a closed system as thermal energy is added
over time.
Temperature (°C)
150°C
100°C.
50°C-
g
0°C-
-50°C
10
20 30 40 50
Time (min)
What happens to the temperature of the water when it begins to melt?
OA The temperature remains at 100°C until the change of state is
complete
B. The temperature continues to increase during the change of state
C. The temperature continues to decrease during the change of
state.
OD. The temperature remains at 0°C until the change of state is
complete.

Answers
Answer:
D. The temperature remains at 0°C until the change of state is complete.
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
1. What was the purpose of the portrait?
2. What is the style of the portrait? (Describe the characteristics of the style presented in the class lecture and Content Page: Renassiance vs Baroque Style.)
3. What is the format of the portrait? (The format of a portrait is bust, half-length, three-quarter length, or full length)
Answers
1. The purpose is that Renaissance portrait focused on the portrayal of the human figure and its realistic representation
2. Artists aimed to depict the natural world accurately, paying attention to details like light, shadow, and texture.
3. The format of the portrait is full length.
How to explain the artThe Renaissance style emerged in Italy during the 14th century and spread throughout Europe during the 15th and 16th centuries. It was characterized by a revival of interest in the art and culture of ancient Greece and Rome. Key features of the Renaissance style include:
Humanism: Renaissance art focused on the portrayal of the human figure and its realistic representation. Artists studied anatomy and perspective to create lifelike and proportionate figures.
Naturalism: Artists aimed to depict the natural world accurately, paying attention to details like light, shadow, and texture. They sought to create a sense of depth and three-dimensionality in their works.
Balance and Harmony: Renaissance artists emphasized the use of balance and harmony in their compositions.
Learn more about portrait on
https://brainly.com/question/29552484
#SPJ1
1. Why the names such as sodium(l) chloride for NaCl and
magnesium(II) chloride for MgCl2 are not used?
Answers
Answer:
It's because the NaCl and MgCl2 is shorter and easier to remember
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Calculate the mass of butane needed to produce 60.6 g of carbon dioxide.
Answers
Answer:
You take the mass of carbon dioxide, 56.8g, divide by its molar mass, 44.01g/mol, to produce the moles of carbon dioxide. This is multiplied by the molar ratio of butane/CO2, (2/8) = 1/4, which gives the moles of butane required to produce the carbon dioxide.
Explanation:
go to google
Water moves on, above or under the surface of the Earth true or false
Answers
above because its above
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
A
QUESTION 4
AEN
Complete Assignment in Spanish
When carbon dioxide concentration went from 800 to 1,000 ppm the temperature in the container went up.
The concentration of carbon dioxide in parts per million (ppm) was measured for a closed container in several situations. Which of the following data collected would demonstrate the action
of photosynthesis?
O When multiple plants were put in the container, carbon dioxide concentration never got above 1.000 ppm.
Plants in the container kept in darkness did not lower the concentration of carbon dioxide below 800 ppm.
QUESTIONS
When the container included a plant exposed to light, carbon dioxide concentration went from 800 to 600 ppm.
Students are provided with a choice of two solutions. Each one is used to test for the presence of a different chemical.
X Close
acer
Save
10
Sign out
May 15
1:58 US
Answers
The data that would demonstrate the action of photosynthesis is:
"When the container included a plant exposed to light, carbon dioxide concentration went from 800 to 600 ppm."
This data shows a decrease in carbon dioxide concentration from 800 to 600 ppm when a plant is exposed to light. During photosynthesis, plants absorb carbon dioxide from the surrounding environment and convert it into glucose and oxygen. The decrease in carbon dioxide concentration indicates that the plant is actively utilizing carbon dioxide for photosynthesis. As a result, the carbon dioxide level in the container decreases, demonstrating the action of photosynthesis. The other provided data points do not specifically indicate the action of photosynthesis. The first data point suggests that multiple plants in the container can prevent the carbon dioxide concentration from exceeding 1,000 ppm, but it does not indicate any decrease in concentration. The second data point mentions plants in darkness, which would not promote photosynthesis.
for more questions on photosynthesis
https://brainly.com/question/20861367
#SPJ11
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
which is generally more soluble in water ammonium chloride or potassium chloride explain
Answers
Answer:
Ammonium chloride
Explanation:
because is a salt which is highly soluble in water than potassium
The cycling of rising and falling magma in the mantle is called a ______________.
A. Convection current
B. Radioactive decay cycle
C. Magnetic oscillation
D. Heat cycle
Answers
Questions: What is required for a compound to be an acid? What's the difference between a strong
acid and a weak acid? What does a compound need to have to be a base? What's the difference
between a strong and a weak base? What does neutralization mean? What's the difference between
pH and pOH? Explain why water can behave like an acid or a base. What will be produced when you
combine an acid with a base? What's the difference between a pH and a pOH scale?
Answers
Answer:
The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water.
1) If a bottle of olive oil contains 1.3 kg of olive oil, what is the volume, in milliliters, of the olive oil? Express your answer to two significant figures and include the appropriate units. 2) A cannon ball made of iron has a volume of 116 cm^3. What is the mass, in kilograms, of the cannon ball? Express your answer to three significant figures and include the appropriate units. 3) A balloon filled with helium has a volume of 6.1 L. What is the mass, in grams, of helium in the balloon? Express your answer to two significant figures and include the appropriate units.
Answers
Answer:
1) 1.4 × 10³ mL
2) 0.913 kg
3) 1.1 g
Explanation:
1)
Step 1: Calculate the volume of 1.3 kg of olive oil
The density of olive oil is 0.917 kg/L.
1.3 kg × (1 L/0.917 kg) = 1.4 L
Step 2: Convert the volume to mL
We will use the relationship 1 L = 10³ mL.
1.4 L × (10³ mL/1 L) = 1.4 × 10³ mL
2)
Step 1: Calculate the mass of the cannon ball
The density of iron is 7.87 g/cm³.
116 cm³ × 7.87 g/cm³ = 913 g
Step 2: Convert the mass to kg
We will use the relationship 1 kg = 10³ g.
913 g × (1 kg / 10³ g) = 0.913 kg
3)
Step 1: Calculate the mass of the helium in the balloon
The density of balloon is 0.18 g/L.
6.1 L × 0.18 g/L = 1.1 g
Which of the following is not soluble in water? E. oil F. salt G. sugar H. honey
Answers
Because oil lacks a charge and is not drawn to the polar water molecules, it is a nonpolar material.
What applications are soluble impurities examples for?The more prevalent soluble contaminants are organic matter, sodium chloride, sodium sulphate, sodium carbonate, magnesium chloride, calcium chloride, calcium sulphate, and calcium bicarbonate. Sand, clay, and organic materials are some common examples of insoluble impurities.
What's soluble and what's not?Solids that may create a solution when combined with the appropriate liquid are said to be soluble (or soluable) solids. Because of this, the soluble substances in the liquid (such as water, oil, kerosene, etc.) dissolve. such as sugar and water or salt and water. Solids that do not dissolve in water are known as insoluble solids.
To know more about soluble visit:-
https://brainly.com/question/29857840
#SPJ1