Which of the following is a problem caused by acid rain?
1 -is a factor in global warming
2-causes depletion of the ozone layer
3-respiratory problems in humans
4-makes lakes and streams unsuitable aquatic life
Answers
Answer:
1
Explanation:
is a factor in global warming
Answer:
sulfur dioxide from coal fired plants in Tennessee
Explanation:
Got it right on the quiz :)
Related Questions
In some regions of the world, forests are cut down and then burned to create room for farmland. How might this local environmental change affect people living on the East Coast of the United States?
A.
It could reduce the amount of carbon dioxide that is released into the air.
B.
It could raise oxygen levels in the ocean, allowing for larger populations of fish.
C.
It could help farms to produce more crops by enriching their soil.
D.
It could contribute to rising temperatures and rising sea levels.
Answers
Those who live on the East Coast of the United States may be affected by environmental change due to rising sea levels and temperatures.
What impact does climate change have on the East Coast?Extreme precipitation events, sea level rise, coastal and riverine flooding, and heat waves are just a few of the effects of climate change that will challenge the Northeast's environmental, social, and economic systems and make its residents, particularly its most vulnerable populations, more vulnerable.
How are humans affected by environmental changes?By affecting the quality of the air and water, spreading some diseases more widely, and changing the frequency or severity of extreme weather events, climate change can also have an effect on human health. Coastal habitats and communities are in danger due to rising sea levels.
To know more about temperatures visit:-
https://brainly.com/question/31055263
#SPJ1
How much heat (in joules) is needed to raise the temperature of 355 g of ethanol (c = 2. 4 J/goC) by 63oC?
Answers
The amount of heat needed to raise the temperature of 355 g of ethanol by 63°C is 53,568 joules (J).
To calculate the amount of heat required to raise the temperature of a substance, we can use the formula:
q = m × c × ΔT
Where:
q = heat energy (in joules)
m = mass of the substance (in grams)
c = specific heat capacity of the substance (in J/g°C)
ΔT = change in temperature (in °C)
Given:
Mass of ethanol (m) = 355 g
Specific heat capacity of ethanol (c) = 2.4 J/g°C
Change in temperature (ΔT) = 63°C
Using the formula, we can calculate the heat energy (q):
q = 355 g × 2.4 J/g°C × 63°C
q = 53568 J
Therefore, the amount of heat needed to raise the temperature of 355 g of ethanol by 63°C is 53,568 joules (J).
learn more about ethanol here
https://brainly.com/question/29294678
#SPJ11
What is the weight of an object that has the area of 74.6 m² and exerts a pressure of 1500 N/m^2
Answers
111900g is the weight of an object that has the area of 74.6 m² and exerts a pressure of 1500 N/m².
Weight being a force The SI unit for weight is Newton (N), which also happens to be the same as the SI unit for force. When we look at how weight is expressed, we can see how it depends on both mass as well as the acceleration caused by gravity; while the mass might not vary from one location to another, the acceleration caused by gravity does.
Pressure = thrust/ area
= weight/ area
1500 = weight/ 74.6
weight = 111900g
To know more about weight, here:
https://brainly.com/question/30176113
#SPJ1
What is the resistance of a 150 W lightbulb connected to a 24 V voltage source?
Answers
Answer:
3.84 Ω
Explanation:
From the question given above, the following data were obtained:
Electrical power (P) = 150 W
Voltage (V) = 24 V
Resistance (R) =?
P = IV
Recall:
V = IR
Divide both side by R
I = V/R
P = V/R × V
P = V² / R
Where:
P => Electrical power
V => Voltage
I => Current
R => Resistance
With the above formula (i.e P = V²/R), we can calculate resistance as illustrated below:
Electrical power (P) = 150 W
Voltage (V) = 24 V
Resistance (R) =?
P = V²/R
150 = 24² / R
150 = 576 / R
Cross multiply
150 × R = 576
Divide both side by 150
R = 576 / 150
R = 3.84 Ω
Thus, the resistance is 3.84 Ω
Calculate the moles of CO2 formed when 4.30 moles of C3H8 reacts with the required 21.5 moles of O2
Answers
Answer : 12.9 moles are formed when 4.30 moles of C3H8 reacts with the required 21.5 moles of O2.
To calculate the moles of CO2 formed when 4.30 moles of C3H8 reacts with the required 21.5 moles of O2, we need to know the balanced chemical equation for the reaction.
C3H8 + 5O2 → 3CO2 + 4H2O
From the balanced chemical equation, we can see that 1 mole of propane reacts with 5 moles of oxygen to produce 3 moles of CO2. Therefore, for 4.30 moles of C3H8 reacting with 21.5 moles of O2, the limiting reactant is C3H8 because the mole ratio of C3H8 to O2 is 1:5.
Hence, the number of moles of CO2 formed is given by the mole ratio of CO2 to C3H8, which is 3:1.Thus, moles of CO2 formed = 4.30 × 3/1 = 12.9 moles.
Know more about mole ratio here:
https://brainly.com/question/15288923
#SPJ11
For hydrogen sulfide at 188 K, H = 2380 J/mol, and S =12.6 J/mol K. Calculate the change in
Gibbs energy. Will the change be spontaneous?
Answers
the change in Gibbs energy is 5.2 J/mol, and the reaction is non-spontaneous under these conditions.
To calculate the change in Gibbs energy, we can use the equation:
ΔG = ΔH - TΔS
ΔH - change in enthalpy,
ΔS - change in entropy,
T - temperature in Kelvin.
at 188 K, ΔH = 2380 J/mol and ΔS = 12.6 J/mol K
ΔG = (2380 J/mol) - (188 K)(12.6 J/mol K)
ΔG = 2380 J/mol - 2374.8 J/mol
ΔG = 5.2 J/mol
The positive value of ΔG indicates that the reactants are more stable than the products and that energy must be added to the system to drive the reaction forward.
Therefore, the change in Gibbs energy is 5.2 J/mol, and the reaction is non-spontaneous under these conditions.
Learn more about Gibbs free energy
https://brainly.com/question/9179942
250.0 mL of a 2.500 M NaOH solution was mixed with 250.0 mL of a 2.500 M HCl solution in a calorimeter. Both the solutions were at the same temperature initially. Determine the heat of the reaction (kJ/mole), if the temperature goes from 2.0 Celsius to 48.8 Celsius. The specific heat of the solution is 4.190 J/goC. Assume a density of 1.025 g/mL.
Please provide it step-by-step. Heat of formation equation = (mass)(specific heat)(change of temp.)
Answers
The reaction has a heat of 161.1 kJ/mol.
What is the molarity of the 250 ml NaOH solution?If 250 mL of a NaOH solution contain 1 milligrams of NaOH, the solution's molarity is 10-4 M. The amount of solute in 1 litre of solution is known as the molarity.
We can use the following formula to get the reaction's heat:
Q = m × c × ΔT
The total volume of the mixture is 500.0 mL because we are aware that the volume of each solution is 250.0 mL:
m = V × ρ
m = 500.0 mL × 1.025 g/mL
m = 512.5 g
The change in temperature must then be calculated:
ΔT = (48.8°C - 2.0°C) = 46.8°C
Assuming that the acid used is HCl and the base used is NaOH, their molar masses are:
HCl: 1 mol of HCl = 36.46 g/mol
NaOH: 1 mol of NaOH = 40.00 g/mol
The reaction between HCl and NaOH has the following chemical formula:
HCl + NaOH → NaCl + H2O
As can be seen, the reaction's stoichiometry is 1:1, which means that 1 mole of HCl combines with 1 mole of NaOH.
So, the following formula can be used to determine the reaction's heat:
Q = m × c × ΔT
Q = 512.5 g × 4.190 J/goC × 46.8°C
Q = 100,697 J or 100.697 kJ
Since both solutions have a 2.500 M molarity, the number of moles of either HCl or NaOH can be determined as follows:
moles = M × V
moles = 2.500 mol/L × 0.2500 L
moles = 0.6250 mol
Therefore, the heat of the reaction is:
Q/mol = Q / moles
Q/mol = 100.697 kJ / 0.6250 mol
Q/mol = 161.1 kJ/mol
To know more about reaction visit:-
https://brainly.com/question/14025220
#SPJ1
an astronaut on the moon throws a ball at a velocity of 4.1 m/s straight up. assuming the gravity on the moon is 1/6 that of earths, how high will the ball reach
Answers
Answer:
(1/2)(1.62) t^2 = 1.4
That will give you the time, t
Explanation:
hope this helps(✿◡‿◡)
The lowest allowable energy state of an atom is called its .
Answers
When
2.0M
aqueous solutions of sodium bicarbonate and hydrochloric acid react, it produces carbon dioxide, water and sodium chloride. Calculate the number of moles of carbon dioxide formed if
31.6 mL
of sodium bicarbonate reacts with excess hydrochloric acid.
15.8 moles CO 2
0.0632 moles CO 2
32.4 moles CO 2
0.0648 moles CO 2
Answers
Option (b) is correct. If 31.6 mL of sodium bicarbonate reacts with excess hydrochloric acid then 0.0632 moles of carbon dioxide formed. This is calculated by using the concept of balanced chemical equation.
The balanced chemical equation for the reaction between sodium bicarbonate and hydrochloric acid can be written as,
Na H CO₃ + H Cl → CO₂ + H₂O + Na Cl
here we can see that 1 mole of sodium bicarbonate produces 1 mole of CO₂.
We have to determine the number of moles of sodium bicarbonate to calculate the number of mole used in the reaction. here concentration and volume of the sodium bicarbonate solution can be used.
The Molarity of sodium bicarbonate solution is 2.0 M.
The Volume of sodium bicarbonate solution used is 31.6 mL = 0.0316 L.
Number of moles of sodium bicarbonate = Molarity x Volume
= 2.0 M x 0.0316 L
= 0.0632 moles
From the balanced chemical equation we get 1 mole of sodium bicarbonate produces 1 mole of CO₂, So, the number of moles of CO₂ produced in the reaction is 0.0632 moles.
To learn more about balanced chemical equation
https://brainly.com/question/14072552
#SPJ4
The correct question is,
When 2.0 M aqueous solutions of sodium bicarbonate and hydrochloric acid react, it produces carbon dioxide, water and sodium chloride. Calculate the number of moles of carbon dioxide formed if 31.6 mL of sodium bicarbonate reacts with excess hydrochloric acid.
A. 15.8 moles CO2
B. 00632 moles CO2
C. 324 moles CO2
D. 0.064 moles CO2
14. A force acts for 0.2 second on a body of mass 80 kg at rest and produces a velocity of 10 ms¹.Find the magnitude of the force.
Answers
The magnitude of the force acting on the body is 4000 Newtons.
To find the magnitude of the force, we can use Newton's second law of motion, which states that the force applied to an object is equal to the product of its mass and acceleration.
The given information includes the mass of the body (80 kg) and the resulting velocity (10 m/s). However, since the time duration (0.2 seconds) is also provided, we can use it to calculate the acceleration of the body.
The formula to calculate acceleration is:
Acceleration = Change in Velocity / Time
The change in velocity can be calculated by subtracting the initial velocity (which is 0 m/s as the body is at rest) from the final velocity:
Change in Velocity = Final Velocity - Initial Velocity
Change in Velocity = 10 m/s - 0 m/s
Change in Velocity = 10 m/s
Now, we can calculate the acceleration:
Acceleration = Change in Velocity / Time
Acceleration = 10 m/s / 0.2 s
Acceleration = 50 m/s²
Finally, we can calculate the magnitude of the force using Newton's second law:
Force = Mass x Acceleration
Force = 80 kg x 50 m/s²
Force = 4000 N
Therefore, the magnitude of the force acting on the body is 4000 Newtons.
for more questions on force
https://brainly.com/question/8106035
#SPJ11
HURRY
What is the volume of a cube with an edge length of 0.843
Answers
Answer:
5.004
Explanation:
............
............
.my Rude g ubddvbrbrbr
sorry in a hurry
Answer: 0.6
Explanation:Yes
A balloon inflated in a room at 24 °C has a volume of 2.00 L. The balloon is then heated to a temperature of 88 °C. What is the volume of the balloon assuming the pressure remains constant?
Answers
Therefore, the volume of the balloon when heated to 88°C is 2.432 L.
What is volume?In the case of a gas, the volume is the amount of space that the gas occupies in a container. The volume of a gas can be affected by changes in pressure and temperature, as described by the ideal gas law. In this case, the volume is often expressed in terms of standard temperature and pressure (STP), which are defined as a temperature of 0°C and a pressure of 1 atmosphere (atm). In chemistry, volume is an important property for understanding the behavior of substances in chemical reactions. The volume of reactants and products can be used to determine the stoichiometry of a reaction, which is the relationship between the amounts of reactants and products in a chemical equation.
Here,
To solve this problem, we can use the ideal gas law, which states that:
PV = nRT
where P is the pressure of the gas, V is its volume, n is the number of moles of gas, R is the universal gas constant, and T is the temperature in Kelvin.
Since the pressure of the gas is assumed to remain constant, we can rearrange the ideal gas law to solve for V:
V1/T1 = V2/T2
where V1 is the initial volume of the balloon, T1 is the initial temperature in Kelvin (24°C = 297 K), V2 is the final volume we want to find, and T2 is the final temperature in Kelvin (88°C = 361 K).
Using this equation, we can plug in the given values and solve for V2:
V1/T1 = V2/T2
2.00 L / 297 K = V2 / 361 K
Multiplying both sides by 361 K, we get:
V2 = 2.00 L x (361 K / 297 K)
V2 = 2.00 L x 1.2162
V2 = 2.432 L
To know more about volume,
https://brainly.com/question/25252629
#SPJ1
Scientists have changed the model of the atom as they
have gathered new evidence. One of the atomic
models is shown below.
What experimental evidence led to the development of
this atomic model from the one before it?
A few of the positive particles aimed at a gold foil
seemed to bounce back.
The colors of light emitted from heated atoms had
very specific energies.
Experiments with water vapor showed that elements
combine in specific proportions.
Cathode rays were bent in the same way whenever
a magnet was brought near them.
Answers
Answer:
C)Equations were used to identify regions around the nucleus where electrons would likely be.
Explanation:
edgenuity 2020
Answer:
Equations were used to identify regions around the nucleus where electrons would likely be.....(C).....
Explanation:
hope this helps. the person above me is correct, the comments were looking for a different question
What conclusion can you draw about the ability of metals to hold on to and attract electrons, as
compared to nonmetals?
Answers
Answer:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction.
Explanation:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction. Also, electrons lost by metals transfer to the nonmetals. It is easier for the metals to lose their valance electrons and form cations rather than gaining electrons.
Metals do not hold on to or attract electrons while nonmetals hold on to or attract electrons.
In the periodic table, metals are found towards the left hand side of the table while nonmetals are found towards the right hand side of the table.
Electron affinity of elements increase from left to right across the period. Electron affinity refers to the ability of elements to attract or hold electrons. This ability increase steadily across the period.
Usually, the electron affinity values of nonmetals are very high showing that they easily hold on to and attract electrons while the electron affinity values of metals is very low showing that they do not easily hold on to and attract electrons.
Learn more: https://brainly.com/question/3964366
what product would form in the grignard reaction of phenylmagnesium bromide with carbon dioxide?
Answers
The product would form in the grignard reaction of phenylmagnesium bromide with carbon dioxide:
\(C_6H_5MgBr\) + \(CO_2\) → \(C_6H_5COOMgBr\) (carboxylate intermediate)
\(C_6H_5COOMgBr\) + \(H_2O\) → \(C_6H_5COOH\) + \(MgBrOH\) (final product)
The reaction of phenylmagnesium bromide (\(C_6H_5MgBr\)) with carbon dioxide (\(CO_2\)) forms benzoic acid (\(C_6H_5COOH\)). The reaction mechanism involves the nucleophilic addition of the carbanion formed by the Grignard reagent to the electrophilic carbon of carbon dioxide to form a carboxylate intermediate. The intermediate is then protonated by water to form the final product, benzoic acid. The reaction can be represented as:
\(C_6H_5MgBr\) + \(CO_2\) → \(C_6H_5COOMgBr\) (carboxylate intermediate)
\(C_6H_5COOMgBr\) + \(H_2O\) → \(C_6H_5COOH\) + \(MgBrOH\) (final product)
Learn more about grignard reaction here https://brainly.com/question/30481814
#SPJ4
This aromatic alcohol and ester containing compound has a molar mass of 152 g/mol and has protons whose integrated peak values are as follows: δ 3.95, s, 3.00 au; δ 6.41-8.30, m, 4.00 au; δ 10.75, t, 0.98 au. We can assume that the peak at δ 0.00 is an internal reference standard of tetramethylsilane and the peak at δ 5.25 is corresponding to water. Therefore, these two peaks can be disregarded from the structural determination. Solve for this unknown compound. Use the integrated values and splitting to support your answer.
Answers
Based on the given information, we know that the compound has a molar mass of 152 g/mol and contains both an aromatic alcohol and an ester functional group.
Looking at the proton NMR spectrum, we can see that there are three distinct peaks: a singlet at δ 3.95, a multiplet from δ 6.41-8.30, and a triplet at δ 10.75. The singlet at δ 3.95 with an integration value of 3.00 au suggests that there are three protons in the same environment, likely attached to an -OH group.
The multiplet from δ 6.41-8.30 with an integration value of 4.00 au indicates that there are four protons in the same environment, likely attached to an aromatic ring. The splitting pattern of the multiplet is not clear from the given information, but we can assume that it is a complex splitting pattern due to the presence of neighboring protons on the ring.
The triplet at δ 10.75 with an integration value of 0.98 au suggests that there is one proton in the same environment, likely attached to an ester group. The triplet splitting pattern indicates that the proton is split by two neighboring protons with a coupling constant (J) of approximately 7 Hz.
Putting all of this information together, we can propose a structure for the unknown compound:
H
|
H - C - O - C - CH3
|
OH
|
Ar-H
The singlet at δ 3.95 corresponds to the three protons on the -OH group, the multiplet from δ 6.41-8.30 corresponds to the four protons on the aromatic ring, and the triplet at δ 10.75 corresponds to the one proton on the ester group. The molar mass of this compound is 152 g/mol, which matches the given information.
Therefore, the unknown compound is likely an aromatic alcohol ester with the proposed structure shown above. The integrated values and splitting pattern support this structure.
Based on the given information, the unknown compound contains an aromatic alcohol and an ester functional group, and has a molar mass of 152 g/mol.
The NMR data provided are:
δ 3.95, s, 3.00 au (aromatic alcohol -OH proton)
δ 6.41-8.30, m, 4.00 au (aromatic ring protons)
δ 10.75, t, 0.98 au (ester -COOCH3 proton)
The aromatic alcohol functional group suggests a phenol derivative, and the ester functional group indicates a -COOCH3 group. The molar mass of 152 g/mol further supports a phenol derivative with an additional ester group.
Taking all these pieces of information into account, the unknown compound is most likely to be methyl 4-hydroxybenzoate (also known as methyl paraben). Its molecular formula is C8H8O3, and its molar mass is 152 g/mol, fitting the given data.
In methyl 4-hydroxybenzoate, the aromatic ring has four protons, which account for the δ 6.41-8.30, m, 4.00 au peak. The -OH proton in the phenol group is represented by the δ 3.95, s, 3.00 au peak, while the -COOCH3 proton is represented by the δ 10.75, t, 0.98 au peak. These integrated values and splitting patterns support the identification of the compound as methyl 4-hydroxybenzoate.
Visit here to learn more about functional group : https://brainly.com/question/14618322
#SPJ11
A compound is found to contain 25.24 % sulfur and 74.76 % fluorine by mass.
What is the empirical formula for this compound?
Answers
Answer:
\(S_{2}\)\(F_{3\\}\), the empical formula
Explanation:
Let's assume we have 100 grams of the compound:
The 100 grams will consist of:
25.24 grams of S
74.76 grams of F
100.0 grams
We need to find the numbers of atoms in each of these masses. Divide each by that element's molar mass to find atoms of each:
Sulfur: (25.24 g)/(32 g/mole S) = 0.789 moles S
Flourine: (74.76 g)/(19.0 g/mole F) = 5.93 moles F
There are 0.8 atom of S for every 6 atoms of F
We can;t have 0.8 atom of anything, so let's multiply it by a factor to make it a whole number. 5 is the best I can find. Both need to be increased by the same factor:
That makes 4 atoms of S and 30 atoms of fluorine.
\(S_{4}\)\(F_{6\\}\)
We can reduce that to \(S_{2}\)\(F_{3\\}\), the empical formula
Can someone please solve this with the working and explanation

Answers
Answer:
A
Explanation:
As you add a bulky group to the end of a carbon chain the boiling point goes up on the molecule.
Again I am here with privious question
I have post this question last time also but no one answers. So can you solve this. I need it.
I will support your account

Answers
Answer:
The answer is below
Explanation:
1. Fractional Distillation
2. Simple Distillation
3. Filtration
4. Steam distillation or lemon grass oil extraction
Difine the term compound
Answers
Answer:
Its when like two pure substances are like combined into one.
Explanation:
People are advised to eat less than 6.00 g of salt (sodium chloride) per day for health reasons.
Which mass of sodium is present in 6.00 g of sodium chloride?
Answers
Answer: 2.36 grams
Explanation: One molecule of NaCl has an atomic mass of 58.4 amu. That consists of one atom each of sodium (23 amu) and chlorine (35.4 amu).
Na 23 (23/58.4) = 0.3934
Cl 35.4 (35.4/58.4) = 0.6066
58.4 amu
For Na: (6 grams)*(0.3934) = 2.36 grams
(e) A 0.050 mol sample of a hydrocarbon was burned in excess oxygen.
The products were 3.60 g of water and 6.60 g of carbon dioxide.
(i) Calculate the number of moles of carbon dioxide produced.
Relative atomic masses: C = 12; O = 16.
Moles of carbon dioxide =
*
(2)
Answers
The correct answer is 0.15.
We are aware that there is 0.05 mol of an unidentified hydrocarbon we will refer to as "X" and that its burning produces 6.6 g of carbon dioxide and 3.6 g of water.
These quantities might be converted to moles by applying the following formula:
amount= mass/ relative atomic mass
Thus, the following equation may be written for H2O: moles = 3.6 / 18 = 0.2 and for CO2: moles = 6.6 / 44 = 0.15.
0.05X + x'O2 = 0.15CO2 + 0.2H2O
This may be made simpler by dividing through by 0.05 (this step is likely to be the most helpful to you), resulting in:
1 x + x O2 = 3 co2 + 4 H2O
The hydrocarbon must have been the source of all the carbon in the carbon dioxide and all the hydrogen in the water.
Accordingly, 4 x 2 = 8 moles of H and 3 x 1 = 3 moles of C.
There are 3/1 = 3 Cs and 8/1 = 8 Hs in one X molecule.
This clearly identifies C3H8 or propane as the hydrocarbon X (dividing by 1 seems unnecessary, but it illustrates the process to use if there were more than one mol of X in the first equation).
To learn more about number of moles of carbon dioxide refer the link:
https://brainly.com/question/12723070
#SPJ9
if the illustration of thomson's atom represents a neutral atom, what must be true about the total amount of positive charge and the total amount of negative charge?
Answers
The illustration of Thomson's atom represents a neutral atom. In this case, the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
What is a neutral atom?A neutral atom is an atom that has no electrical charge. An atom is neutral because it has the same amount of positively charged protons and negatively charged electrons. The nucleus of an atom contains protons, which are positively charged particles. Electrons, which are negatively charged particles, are located in the atom's electron cloud around the nucleus.
Electrons, protons, and neutrons are the three components of atoms. Electrons are negatively charged, protons are positively charged, and neutrons have no charge. Electrons are found outside the nucleus of the atom and are continually moving at high speeds.
In summary, if the illustration of Thomson's atom represents a neutral atom, then the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
Learn more about Thomson's atom on the given link:
https://brainly.com/question/1597441
#SPJ11
What is the purpose of adding base in the aldol condensation reaction? Choose the best answer. a Protonate a-carbon to generate electrophile b To generate intermediate enol c Deprotonate a-carbon to generate electrophile d Protonate a-carbon to generate nucleophile e Deprotonate a-carbon to generate nucleophile f To neutralize acid
Answers
The purpose of adding base in the aldol condensation reaction is c) Deprotonate α-carbon to generate electrophile.
What is aldol condensation?
Aldol condensation is a reaction in organic chemistry that involves the condensation of two carbonyl compounds, typically an aldehyde and a ketone, to form a β-hydroxy carbonyl compound.
In the aldol condensation reaction, a base is added to deprotonate the α-carbon of the carbonyl compound, typically an aldehyde or a ketone. The deprotonation of the α-carbon generates an enolate ion, which is an excellent nucleophile. This deprotonation step is crucial in generating the reactive electrophile necessary for the aldol condensation reaction.
By deprotonating the α-carbon, the base increases the electron density on the carbon atom, making it more nucleophilic and prone to react with another carbonyl compound. This enables the formation of a new carbon-carbon bond, resulting in the formation of an aldol product.
Therefore, the purpose of adding a base in the aldol condensation reaction is to deprotonate the α-carbon and generate an electrophilic enolate ion, which can then react with another carbonyl compound to form the desired product.
To know more about aldol condensation, refer here:
https://brainly.com/question/31558115
#SPJ4
Count the atoms in this common formula for the explosive TNT
2C7H5(NO2)3
Answers
Answer:
Explanation:
7 carbon atoms, 6 hydrogen atoms,9 NO2 atoms
7+6+9=22
2(22)=44
44 atoms
What is the pH of a 1.0 x 10-4 M solution of KOH?
Answers
Answer:
pH = 10
Explanation:
KOH is a strong base; thus it will completely dissociate:
KOH -> K⁺ + OH⁻
Since it completely dissociates, the concentration of both K⁺ and OH⁻ will be the concentration of KOH given (1.0 x 10^-4 M).
We can then find pOH by taking the negative log of the OH⁻, or hydroxide, concentration:
pOH = -log[OH⁻] = -log[1.0 x 10^-4 M] = 4
At 25 degrees Celsius, pH + pOH = 14. If we solve for pH and then plug in our pOH, we get:
pH = 14 - pOH = 14 - 4 = 10
The pH of a 1.0 x 10^-4 M solution of KOH is thus 10.
Temperature (average kinetic energy) affects the density of a substance.
True
False
Answers
Answer:
True
Explanation:
Higher temperature will have greater volumes so the density will be less.
Karmina inflated a balloon at sea level, where the atmospheric pressure was 101. 3 kilopascals. The volume of the balloon was 2. 15 liters. She then carried the balloon with her to the top of a mountain, where the atmospheric pressure was 83. 1 kilopascals. If the temperature was constant and no air leaked out of the balloon, what was the balloon’s volume at the top of the mountain?.
Answers
The volume of the balloon at the top of the mountain can be calculated using Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at a constant temperature.
The formula for Boyle's Law is P1V1 = P2V2, where P1 is the initial pressure, V1 is the initial volume, P2 is the final pressure, and V2 is the final volume.
We are given the initial pressure (P1) as 101.3 kilopascals, the initial volume (V1) as 2.15 litres, and the final pressure (P2) as 83.1 kilopascals. We need to find the final volume (V2).
Plugging the given values into the formula, we get:
101.3 kPa × 2.15 L = 83.1 kPa × V2
Rearranging the equation to solve for V2, we get:
V2 = (101.3 kPa × 2.15 L) / 83.1 kPa
V2 = 217.795 L / 83.1 kPa
V2 = 2.62 L
Therefore, the volume of the balloon at the top of the mountain is 2.62 litres.
You can learn more about volume at: brainly.com/question/13338592
#SPJ11
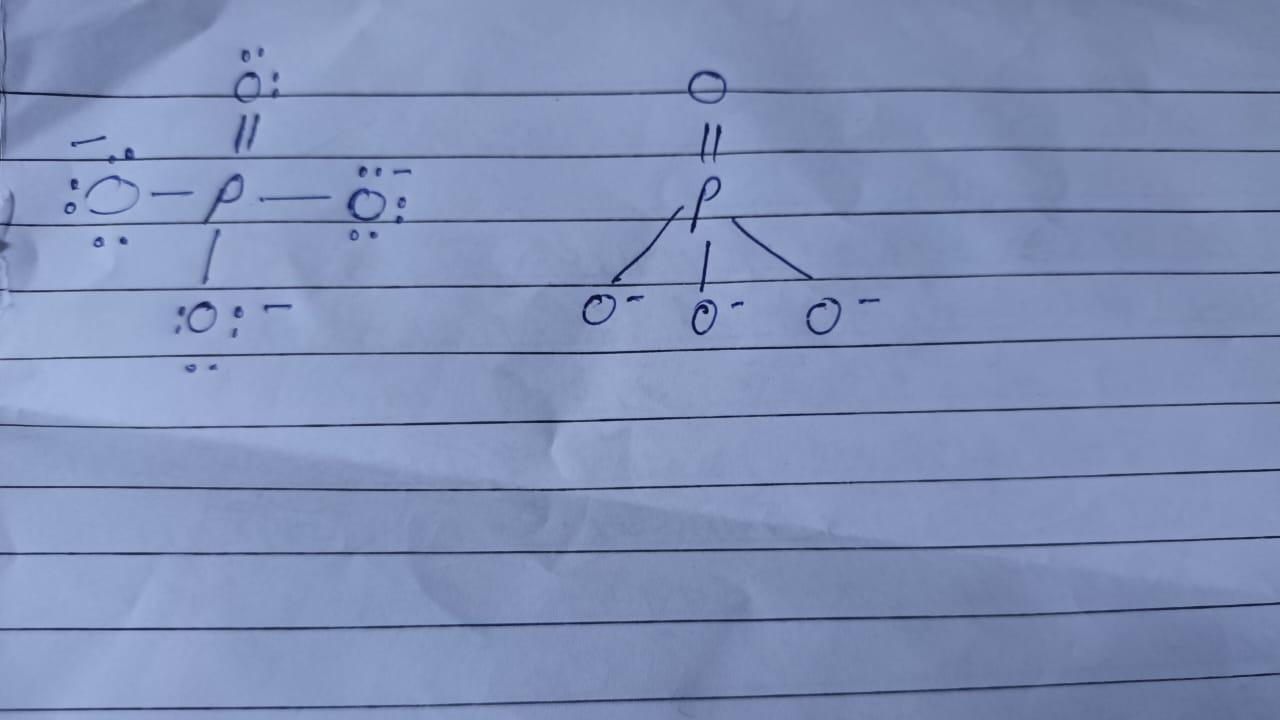
PO4 3‒ 1. Lewis Structure 2. Perspective drawing 3. Number of atoms bonded to central atom 4. Number of non-bonding electron pairs on the central atom 5. Electronic geometry: 6. Molecular geometry with ideal bond angles 7. Hybridization of central atom 8. Symmetric or asymmetric?
Answers
PO4³⁻ is a symmetric molecule having Sp3 hybridisation.
Hybridization, as related to genomics, is the procedure wherein complementary unmarried-stranded DNA and/or RNA molecules bond collectively to form a double-stranded molecule.
Hybridisation is a idea of blending atomic orbitals into new hybrid orbitals which might be generally of lower electricity and suitable for the pairing of electrons to shape chemical bonds in valence bond theory.
1.Lewis Structure = PO4³⁻ image below
2.Perspective drawing = image below
3. Number of atoms bonded to central atom = 4 oxygen atom ( O)
4. Number of non-bonding electron pairs on the central atom = O since P uses all its 5 electrons in forming 5 bonds within the four oxygen.
5. Electronic geometry = Tetrahedral
6. Molecular geometry with ideal bond angles = Tetrahedral with 109.5 degree bond angles.
7. Hybridization of central atom = Sp3 since hybridisation = number of sigma bonds + lone pairs on central atom. Here hybridisation = 4 + 0 = 4 i.e 1's' and 3 'p' orbital are used in bonding So, Sp3
8. Symmetric or asymmetric = Symmetric
Learn more about Lewis Structure here:-https://brainly.com/question/6224949
#SPJ4