when the concentrations of both mercury chloride and oxalate ion are 0.30 m, what is the rate of the reaction?
Answers
The concentrations of both mercury chloride and oxalate ion are 0.30 m, the rate of the reaction is 7.8 × 10⁻⁴ M/s.
given that :
concentration of mercury chloride = 0.30 M
concentration of oxalate ion = 0.30 M
It can be written as:
2HgCl₂ + C₂O₄²⁻ -----> 2Cl⁻ + Hg₂Cl₂ + 2CO₂
k = 8.7 × 10⁻³ M⁻²/s⁻¹
The rate of the reaction will be shown as :
Rate = k [HgCl₂ ] [ C₂O₄²⁻ ]²
Rate = 8.7 × 10⁻³ (0.30)(0.30)²
Rate = 7.8 × 10⁻⁴ M/s
Thus, rate of the reaction will be :
Rate = 7.8 × 10⁻⁴ M/s
This question is incomplete , the complete question is :
__""when the concentrations of both mercury chloride and oxalate ion are 0.30 m, what is the rate of the reaction? "__
the rate is given as : Rate = k[HgCl₂ ] [ C₂O₄²⁻ ]² value of k = 8.7 × 10⁻³ M⁻²/s⁻¹__"
To learn more about rate of the reaction here
https://brainly.com/question/14202389
#SPJ4
Related Questions
Emissions of sulfur dioxide by industry set off chemical changes in the atmosphere that result in acid rain. The acidity of liquids is measured by pH on a scale from 0 to 14. Distilled water has a pH of 7.0 and lower pH values indicate acidity. Theory suggests that the pH of rain varies among rainy days according to a normal distribution with a mean of 5.4 and a standard deviation of 0.5. The random sample of 21 days gives a sample standard deviation of 0.8. You would like to test if the population standard deviation is indeed 0.5 as the theory suggests. At alpha equals 0.05, what is the test statistic and what are the critical values? The test statistic: 53.76. Critical values: 9.591 and 34.170. The test statistic: 53.76. Critical values: 10.283 and 35.479. The test statistic: 51.20. Critical values: 10.283 and 35.479. The test statistic: 51.20. Critical values: 9.591 and 34.170.
Answers
The main answer to the question is: The test statistic is 51.20 and the critical values are 9.591 and 34.170.
To explain the main answer, we are conducting a hypothesis test to determine if the population standard deviation of the pH of rain is indeed 0.5, as suggested by the theory. The null hypothesis (H0) is that the population standard deviation is 0.5, while the alternative hypothesis (H1) is that the population standard deviation is not 0.5.
In this case, we are given a random sample of 21 rainy days, and the sample standard deviation is 0.8. To test the hypothesis, we need to calculate the test statistic, which is given by the formula: test statistic = [(sample standard deviation) - (hypothesized standard deviation)] / (sample standard deviation / sqrt(sample size)).
Plugging in the values, we get: test statistic = [(0.8 - 0.5) / (0.8 / sqrt(21))] = 51.20.
To determine the critical values, we need to look at the critical region associated with the given significance level (alpha) of 0.05. Since this is a two-tailed test, we divide the significance level by 2, resulting in an alpha of 0.025 for each tail. Using the degrees of freedom (n-1), which is 20 in this case, we can consult the t-distribution table or use a statistical software to find the critical t-values. For an alpha of 0.025 and 20 degrees of freedom, the critical t-values are approximately ±2.093.
Converting the t-values to critical values using the formula: critical value = (hypothesized standard deviation) + (t-value * (sample standard deviation / sqrt(sample size))), we get: critical values = 0.5 + (2.093 * (0.8 / sqrt(21))) = 9.591 and 0.5 - (2.093 * (0.8 / sqrt(21))) = 34.170.
Therefore, the correct answer is: The test statistic is 51.20 and the critical values are 9.591 and 34.170.
Learn more about: Standard deviation
brainly.com/question/13498201
#SPJ11
chemistry balance the reactions

Answers
The balanced equation of the reaction would be: \(Cu + 2AgNO_3 --- > 2Ag + Cu(NO_3)_2\)
Balancing chemical equationsA balanced chemical equation will have an equal number of atoms of different elements in the reactants and in the products. In other words, a balanced chemical equation will obey the law of conservation of atoms.
Thus, the balanced chemical equation of the reaction shown in the illustration would be:
\(Cu + 2AgNO_3 --- > 2Ag + Cu(NO_3)_2\)
The atoms of different elements in the reactants balance the numbers in the products.
More on balancing chemical equations can be found here: https://brainly.com/question/14072552
#SPJ1
If a neutral atom has 24 electrons and 26 neutrons how many protons does it have?
Answers
Answer:
24
Explanation:
The number of protons in the atom of the neutral element will also be 24.
Protons are the positively charged particles in an atom.
Electrons are the negatively charged particles
Neutrons do not carry any charges at all
Now, in a neutral atom, the charge is balanced and the number of protons and electrons are the same in the atom.
So, since we have been given that the atom has 24 electrons, the number of protons will be 24.
Calculate the H3O+/OH- ratio for solutions of your choice using all three values: the Molecule count, Quantity of moles, and Concentration Show your work
-water
-one base
-one acid
Answers
H3O+ and OH- ion concentrations in water are equivalent at 25 °C and have a value of 1.0 x 10-7 M each.
What is the bond between OH and H3O+?In general, a material will be an acid if [H3O+] > [OH]. Moreover, the substance will be a base if [OH] > [H3O+]. We are aware that neutral compounds do exist, though. When this occurs, [H3O+] = [OH].
What are the pH and hydronium ion concentrations of pure water at 25 C?Kw equals 1.011014 at 25 °C, therefore pH+pOH=pKw=14.00. Because the reaction is endothermic, the concentrations of the hydronium ion and hydroxide ion that occur from ionisation grow with temperature.
To know more about concentrations visit:-
https://brainly.com/question/10725862
#SPJ1
What are the three states of matter, describe atoms during these states.
Answers
Answer:
Three states of matter is liquid, solid and gas.
I haven't learned atoms, I just learned the states in 5th grade.
Answer:
the three states of matter are
1. solid ( atoms are closely packed )
2. liquid ( atoms are loosely packed )
3. gas ( atoms are very loosely packed )
a 15.79 g sample of a hydrate of an iron(ii) sulfate compound was heated, without decomposing the sulfate to drive off the water. the mass was reduced to 8.63 g . what is the formula of the hydrate?
Answers
The formula of the hydrated salt would be \(FeSO_4.7H_2O\)
Stoichiometric problemThe formula of the hydrated salt of iron (II) sulfate would be in the form, \(FeSO_4.xH_2O\). The number of moles of water of hydration can be determined using the empirical formula approach.
Mass of hydrated salt = 15.79 g
Mass of anhydrous salt = 8.63 g
Mass of water of hydration = 15.79 - 8.63
= 7.16 g
Now, let's find the equivalent mole of each component of the hydrated salt.
Molar mass of iron (II) sulfate = 151.908 g/mol
Molar mass of water of hydration = 18.01 g/mol
Mole of iron (II) sulfate = 8.63/151.908
= 0.0568
Mole of water of hydration = 7.16/18.01
= 0.3976
Divide by the smallest mole:
Iron (II) sulfate = 0.0568/0.0568 = 1
Water of hydration = 0.3976/0.0568 = 7
In other words, x, which represents the number of moles of water of hydration, is 7.
Hence, the formula of the hydrated salt would be \(FeSO_4.7H_2O\).
More on the formula of hydrated salts can be found here: https://brainly.com/question/18264811
#SPJ1
a compound forms a dense yellow precipitate when treated with iodine and sodium hydroxide. the compound must be:
Answers
A compound forms a dense yellow precipitate when treated with iodine and sodium hydroxide. the compound must be: Acetophenone methyl ketone.
Sodium hydroxide is every now and then known as caustic soda or lye. it's by far a common ingredient in cleaners and soaps. At room temperature, sodium hydroxide is white, odorless strong. Liquid sodium hydroxide is colorless and has no odor. it could react violently with strong acids and with water.
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. it is a white stable ionic compound that includes sodium cations Na⁺ and hydroxide anions OH⁻.
Manufacturers may additionally use sodium hydroxide to provide soaps, rayon, paper, products that explode, dyes, and petroleum merchandise. different obligations which can use sodium hydroxide include processing cotton cloth, steel cleaning and processing, oxide coating, electroplating, and electrolytic extraction.
Learn more about sodium hydroxide here:-https://brainly.com/question/25597694
#SPJ4
determine the value of k so that the damping ratio is 0.6. also obtain the peak time, maximum overshoot and settling time for the step response.
Answers
Therefore, the value of k is 0.64/T², peak time is 1.25T, maximum overshoot is 23.6% and settling time is 6.67 seconds.
Given data:
Damping ratio, ζ = 0.6
We know that the settling time, Ts = 4 / ζωn
The formula for the natural frequency is given as follows;ωn = √(1 - ζ²) / Tk = 2π / T
Therefore, substituting the values of k and ζ in the equations, we have:
ωn = √(1 - ζ²) / Tωn = √(1 - (0.6)²) / Tωn = √(0.64) / Tωn = 0.8 / T
T = 2π / ωnk = ωn²Then; k = (0.8 / T)²
k = 0.64 / T²
We have also, Ts = 4 / ζωnTs = 4 / (0.6 * 0.8 / T)Ts = 6.667 seconds
Maximum overshoot (Mp) is given by;
Mp = e^(-πζ / √(1 - ζ²))
Mp = e^(-π * 0.6 / √(1 - 0.6²))Mp = 0.236
Settling time (Ts) is given by;
Ts = 4 / ζωnTs = 4 / (0.6 * 0.8 / T)Ts = 6.667 seconds
Therefore, the value of k is 0.64/T², peak time is 1.25T, maximum overshoot is 23.6% and settling time is 6.67 seconds.
To know more about damping ratio visit:
https://brainly.com/question/20115234
#SPJ11
a compound was analyzed and found to contain 12 grams of carbon, 2 grams of hydrogen and 16 grams of oxygen, what is the empirical formula for this compound?
Answers
The empirical formula of the compound containing 12 grams of carbon, 2 grams of hydrogen and 16 grams of oxygen is CH2O
To determine the empirical formula of a compound, one needs to know the relative amounts of each element in the compound. In this case, we are given that the compound contains 12 grams of carbon, 2 grams of hydrogen, and 16 grams of oxygen.
The first step is to convert the masses of each element into moles by dividing each by its molar mass. The molar mass of carbon is 12 g/mol, hydrogen is 1 g/mol, and oxygen is 16 g/mol. Therefore, we have:
- Carbon: 12 g / 12 g/mol = 1 mol
- Hydrogen: 2 g / 1 g/mol = 2 mol
- Oxygen: 16 g / 16 g/mol = 1 mol
Next, we need to find the simplest whole number ratio of the atoms in the compound. This is done by dividing each mole value by the smallest mole value. In this case, the smallest mole value is 1, so we divide all mole values by 1:
- Carbon: 1 mol / 1 = 1
- Hydrogen: 2 mol / 1 = 2
- Oxygen: 1 mol / 1 = 1
Therefore, the empirical formula of the compound is CH2O, which represents the simplest whole number ratio of the atoms in the compound.
For more such questions on empirical formula, click on:
https://brainly.com/question/1603500
#SPJ11
The direction of movement of the cold front shown in the figure at left is _____.
northeast
southeast
southwest
northwest

Answers
Answer:
Its southeast
Explanation:
Answer:
southeastt
Explanation:
What type of compound are carbon tetrachloride and boron trihydride?
Answers
Answer:
hydrocarbon
Explanation:
these are called hydrocarbons
Fill in the missing values in the chart below please. If you can please show your work.

Answers
The volume and temperature changes in according to Charles' law will be:
T2 = 363 °CT1 = 869 KV1 = 5.35 LV2 = 144.6 mLWhat is the relationship between volume and temperature of a given mass of gas?The relationship between the volume and temperature of a given mass of gas is given by Charles' Iaw which states that volume is directly proportional to temperature if a gas in Kelvin.
\(Mathematically, V_1/T_1 = V_2T_2\)To complete the tables volume and temperature changes:
T2 = 3.5 × (273 +45)/1.75 = 636 K = 363 °CT1 = 35 × 298/12 = 869 KV1 = 250 × 7.6/355 = 5.35 LV2 = 150 × (273 + 24)/(273 + 35) = 144.6 mLTherefore, volume and temperature are directly proportional.
Learn more about gas Charles' law at: https://brainly.com/question/888898
when and if element 119 is discovered predict where it would be placed on the modern periodic table
Answers
Answer:yes
Explanation:
Because modern periodic table is designed in such a way that it can accomodate any new elemet which may be discovered in future
We have that for the Question "when and if element 119 is discovered predict where it would be placed on the modern periodic table" it can be said that With the existing information the element 119 will most likely be i the 8th period and the first group of the periodic table
8th period and the first group of the periodic tableFrom the question we are told
when and if element 119 is discovered predict where it would be placed on the modern periodic table
Generally
The Periodic table is naturally designed to accommodate new elements based on there properties
Therefore
With the existing information the element 119 will most likely be i the 8th period and the first group of the periodic table
For more information on this visit
https://brainly.com/question/17756498
Antibiotics are powerful medicines for treating some infections, but they have limitations. while antibiotics are very effective in the treatment of bacterial infections, they do not work against viral infections. what differences between bacteria and viruses account for this?
Answers
Differences between bacteria and viruses is that viruses do not have cell wall.
Only bacterial infections can be treated with antibiotics, viruses do not respond to antibiotics.
Antibiotics attack the cell wall or coating surrounding bacteria. There are two types of cell wall: Gram-positive and Gram-negative.
For example antibiotic penicillin:
After it was discovered, penicillin was very effective in destroying the bacterium Staphylococcus aureus. Today, because of natural selection in Staphylococcus aureus populations, they are resistant to antibiotics based on penicillin (Staphylococcus aureus bacterium is resistant to methicillin, β-lactam antibiotic of the penicillin class).
Staphylococcus aureus is a Gram-positive, round-shaped bacterium which causes a skin infections.
More about bacteria: brainly.com/question/6941760
#SPJ4
the lewis dot structure rule states that S=N-A. recall that A represents the TOTAL number of valence electrons available from all the atoms in molecule. what is A from silicon tetrachloride, SiCL4
Answers
Answer: oh i thought i knew it nvm im sorry love
Explanation:
A, the total number of valence electron is 24 for Silicon Tetrachloride , SiCl₄ .
What is Lewis Dot Structure ?In Lewis Dot structure S=N-A is used to calculate the total number of shared and unshared electrons in a molecule.
S is the number of shared electrons ,N is the number of total valence shell electrons required by all the atom of the molecule, A is the total number of valence electrons available from all the atoms in the molecule
For SiCl₄,
• We have to first count the valence electron on each atom that is coming to form a molecule .
Valence electron on Si = 4
Valence Electron on Cl = 7
Total valence electron on SiCl₄ = 4+ 4 * 7 = 32
• Then we find the least electronegative atom in the molecule and place it at the center , In SiCl₄ , Si has 1.8 and Cl has 3.16 and so Si is placed at the center and four Cl atoms are connected with a single bond
• A single bond takes up 2 valence electron and so for four bonds 8 valence electrons have been occupied , so we are left with 32-8=24 valence electrons.
Therefore In the Lewis Dot structure S=N-A , Total number of valence electrons available from all the atoms in molecule of SiCl₄ is 24.
To know more about Lewis Dot structure : https://brainly.com/question/20300458
#SPJ2

A serum sample drawn in the emergency room from a 42-year-old man yielded the follow¬ing laboratory results: CK 185 Units (Normal = 15-160) AST 123 Units (Normal = 0-48) CK-MB 6 Units (Normal = 2-12) Which of the following conditions might account for these values?
a) crush injury to the thigh
b) cerebrovascular accident
c) pulmonary inhrction
d) early acute hepatitis
Answers
The CK levels were raised in the serum sample taken from the patient, indicating that there was muscle damage. This could have resulted from a crush injury to the thigh. Hence, option a) is correct.
Serum samples from patients are drawn in emergency rooms to check for their enzyme and protein levels, particularly to verify if they've had a heart attack.
CK, AST, and CK-MB are the most commonly used tests.
A crush injury to the thigh may account for these values. This is because a crush injury to the thigh can result in muscle damage and increased levels of creatine kinase (CK) in the blood.
The CK enzyme is found in skeletal muscle, cardiac muscle, and brain tissue. It can be released into the bloodstream as a result of muscular damage.
The CK levels were raised in the serum sample taken from the patient, indicating that there was muscle damage. This could have resulted from a crush injury to the thigh. Hence, option a) is correct.
Pulmonary infarction is caused by an obstruction in the pulmonary artery.
AST and CK-MB levels aren't affected by pulmonary infarction, so option c) is incorrect.
Cerebrovascular accident, on the other hand, is caused by an obstruction in the blood supply to the brain. It doesn't have an effect on CK, AST, and CK-MB levels, so option b) is incorrect.
Early acute hepatitis, the final option, also doesn't have an effect on CK, AST, and CK-MB levels, so option d) is incorrect.
The correct option is a) crush injury to the thigh.
To know more about CK levels, visit:
https://brainly.com/question/30327287
#SPJ11
what is the mass of iron
Answers
Atomic mass: 55.845
^ Atomic Mass of the element Iron
~theLocoCoco
An ecosystem is all the populations of organisms that live together and
A.
the food that they take in as energy.
B.
the organisms that generate energy from sunlight.
C.
the microorganisms that depend on them.
D.
the physical factors with which they interact.
Plllzzzzz help :(
Answers
Answer:
D i belive
Explanation:
Why are scientific models important in the study of science?
Answers
Answer:
In science, amodel is a representation of an idea, an object or even a process or a system that is used to describe and explain phenomena that cannot be experienced directly. Models are central to what scientists do, both in their research as well as when communicating their explanations.
Explanation:
Answer:
They always involve critical mathematical calculations.
Explanation:
tell me if im wrong but this one makes the most since to me
Why is the reactivity of metal increase but that of non-metal decreases as we go down in a group of periodic table? (pls its important i need this question's ans for my exam so answer it fast thank you)
Answers
Answer:
Nonmetal reactivity decreases down a group because the nucleus' ability to gain more valence electrons weakens due to more nuclear shielding. For Metals: the most reactive metals are those that can lose their valence electrons the most easily. ... Francium is the most reactive metal
Explanation:
Answer:
While moving from top to bottom in a group of the periodic table, the reactivity of non- metals decreases. ... Thus, the tendency of gaining electron/s in the valence shell decreases as well as the chemical reactivity also decreases on moving from top to bottom in a group of non- metals.
Which response is false? An acid-base indicator ____.
a. might be an acid
b. might have only one highly colored form
c. might be a base
d. might have two highly colored forms
e. can be non-polar
Answers
The false statement among the options is An acid-base indicator can be non-polar. Option e is correct answer.
An acid-base indicator is a substance that undergoes a color change in the presence of an acid or a base. It is typically a weak acid or a weak base that can exist in different forms, each having a different color. When an indicator is in its acidic form, it may be represented as an acid (option a) and have a specific color. Similarly, when it is in its basic form, it can be considered as a base (option c) and exhibit a different color. Therefore, options a and c are true statements.
Furthermore, an indicator can have one highly colored form (option b) or two highly colored forms (option d), depending on its acid-base equilibrium and the pH of the solution. For example, litmus is a commonly used indicator that exists in two forms: red in acidic solutions and blue in basic solutions.
However, the statement in option e, that an acid-base indicator can be non-polar, is false. Acid-base indicators are typically polar compounds because they contain functional groups that are involved in acid-base reactions. The polar nature of the indicator molecules allows them to interact with polar solvents and participate in the necessary chemical reactions for color changes.
Learn more about acid-base indicator here
https://brainly.com/question/13004359
#SPJ11
1. X is a crystalline salt.On exposure to air ,there is a loss in mass of x (a)suggest a phenomenon exhibited by x. (b)mention 2 salts that x could be. 2 a)Define salt hydrolysis b)mention 2 examples of salts that produce alkaline solutions when dissolve in water.
Answers
Answer:
1. a) Crystalline hydrated salt lose their mass or moisture on exposure to the air and change into amorphous salt, the phenomenon is called efflorescence.
b) Two salts that x could be are gypsum (Caso4.H2O) and copper (II) sulfate (CuSO4.H2O).
Gypsum (Caso4.H2O) form anhydrite(CaSO4), when exposed to air and copper (II) sulfate (CuSO4.H2O) form a white layer of anhydrous copper (II) Sulfate when expose to sir.
2. a) Salt hydrolysis is defined as the ions from salts react with water and form either basic or an acidic solution.
b) Two examples of salts that produce alkaline solutions when dissolving in water are sodium bicarbonate (NaHCO3) and Calcium Carbonate (CaCO3).
how many atoms are in FeS₂?
Answers
Surface model,we used a supercell of
16.1106×16.1106×18.0 And a model of a 3 × 3
9 atoms..
if this answer helps you plz mark as brainlist..But, don't report my answer..How many compounds with the formula C7H16 (heptanes) contain a single 3° carbon atom?
a) 2
b) 3
c) 4
d) 5
e) 6
Answers
There are no compounds with the formula C₇H₁₆ that contain a single 3° carbon atom because each of the seven carbon atoms in heptane is either a 1° or a 2° carbon atom.
To determine the number of compounds with the formula C₇H₁₆ that contain a single 3° carbon atom, we must first understand what a 3° carbon atom is. A 3° carbon atom is a carbon atom that is bonded to three other carbon atoms, meaning that it is located in the middle of a chain of carbon atoms.
With this in mind, we can begin to count the number of compounds with a single 3° carbon atom. There are seven carbon atoms in the heptane molecule, so we must consider each carbon atom, in turn, to see if it is a 3° carbon atom.
Starting with the first carbon atom in the chain, we see that it is bonded to two other carbon atoms. This means that it is a 2° carbon atom and not a 3° carbon atom. Moving on to the next carbon atom, we see that it is also bonded to two other carbon atoms, so it is also a 2° carbon atom.
Continuing down the chain, we find that each of the seven carbon atoms is either a 1° or a 2° carbon atom. Therefore, there are no compounds with the formula C₇H₁₆ that contain a single 3° carbon atom.
In summary, there are no compounds with the formula C₇H₁₆ that contain a single 3° carbon atom because each of the seven carbon atoms in heptane is either a 1° or a 2° carbon atom.
To know more about carbon atoms refer here:
https://brainly.com/question/15325677#
#SPJ11
Please Help !! Brainliest !!

Answers
Data supports significantly because we can use the testing(depending sample) before and after we use the same object to test the hypothesis.
What type of reaction do Carbon -14 and Uranium- 238 undergo? Explain how you figured this out and write the reaction for each
Answers
The reaction for Carbon-14, used in carbon dating, decays by beta emission and in Uranium-238 decays by alpha emission.
Alpha radiation releases when the nucleus of an atom becomes unstable and alpha particles are released in order to restore stability. Alpha decay occurs in elements have high atomic numbers, such asuranium, radium, and thorium etc. The reaction that describes an alpha emission because radiations are 5740 years. Now, Carbon-14 has a half life of 5730 yrs, and it used to date fossils of 50 hundred yrs old. It undergo beta emission. In case of Uranium- 238, has half life of 236 yrs. Because there is so much difference between half lives of both so we can't use both of together in one reaction. So, it goes on alph emission. The reactions are
¹⁴₆C → ¹⁴₇N - e⁻
Hence, required reaction is alpha emmision.
For more information about alpha emission, visit :
https://brainly.com/question/27875918
#SPJ4
Please Help
Predict the nature of the indicated covalent
bond. Polar or Non polar
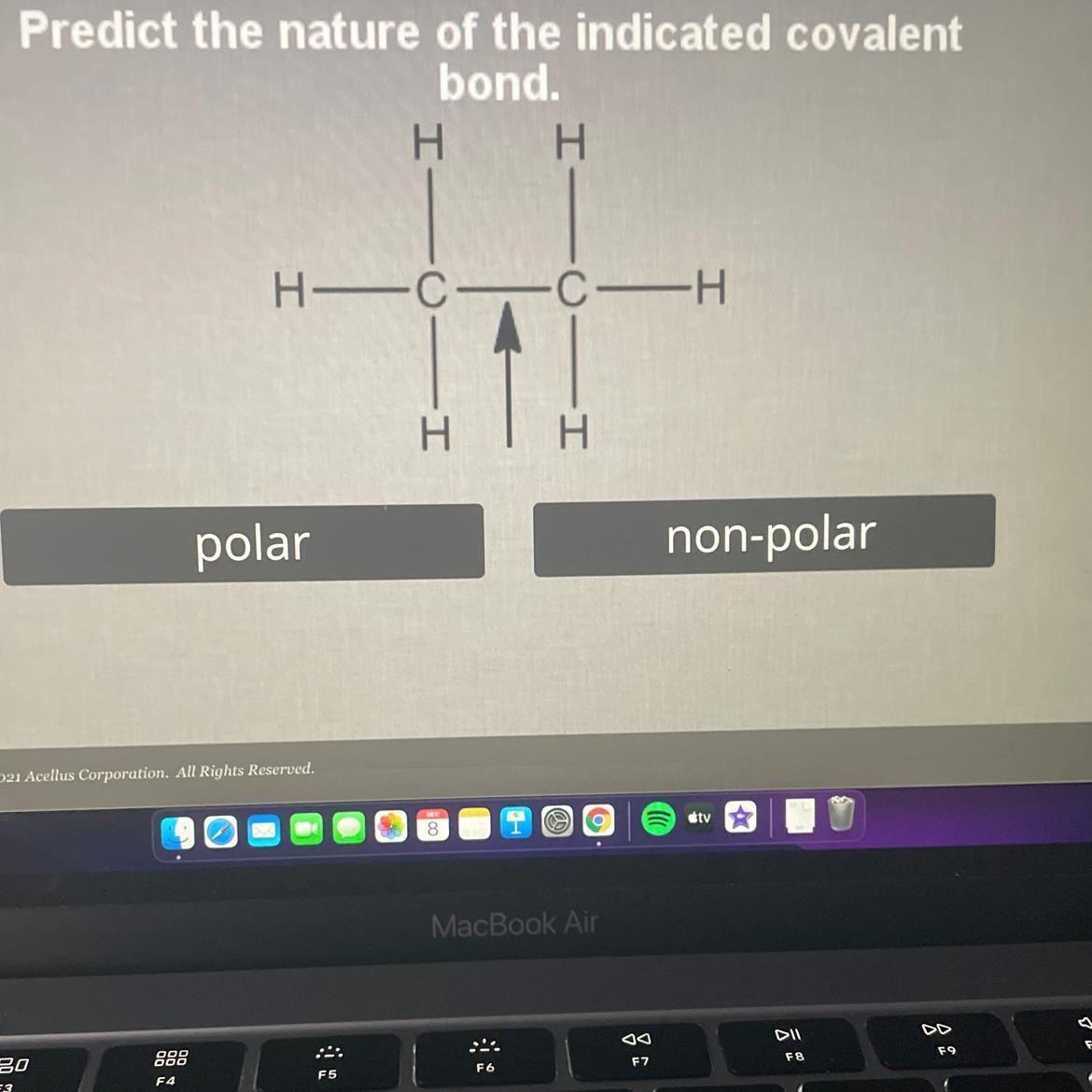
Answers
Answer:
Nonpolar
Explanation:
6. During a phase change (e.g., the transition from solid to liquid), significant energy is absorbed by the system. Yet, the temperature remains constant. Where does the energy go
Answers
Explanation:
The energy absorbed by the system during phase change is referred to as latent heat.
Latent heat is the energy absorbed or released by a body during a phase change without change in its temperature.
The energy is what is being used for the phase change and cannot be measured by the thermometer.
can someone give me facts about Venus, it's for a project
it would help a lot!!
I'm looking for at least 4
Answers
Answer:
A day on Venus is longer than a year. ...
Venus is hotter than Mercury – despite being further away from the Sun. ...
Unlike the other planets in our solar system, Venus spins clockwise on its axis. ...
Venus is the second brightest natural object in the night sky after the Moon.
Venus has a hostile environment. ...
Venus is hellishly hot. ...
Venus has volcanic features. ...
Venus has year-long days. ...
Venus has two sunrises in a year. ...
Venus spins in reverse gear. ...
Venus is showing mysterious life signals.
Explanation:
hope it helps
Please rate and mark as brainliest
Thank you and have a good day
A day on Venus is longer than a year. ...
Venus is hotter than Mercury – despite being further away from the Sun. ...
Unlike the other planets in our solar system, Venus spins clockwise on its axis. ...
Venus is the second brightest natural object in the night sky after the Moon.
Venus has a hostile environment
In a given experiment, 0.10 atm of each component in the following reaction is placed in a sealed container. In which direction will the reaction proceed
Answers
The reaction will proceed towards right hand side and hence it will encourage product formation.
The reaction quotient or Q is the ratio of product to reactant at any time during the reaction. In stated case, the reaction will be -
Qp = {(0.10)×(0.10)²}/(0.10)²
Performing multiplication and division
Qp = 0.1
The value of Qp is 0.1 while given value of Kp is 60.6.
Now, Q represents the reaction quotient while K represents the equilibrium constant. If the value of reaction quotient is less than equilibrium constant, the reaction towards right side. This tends to increase product formation and hence the reaction proceeds to right hand side.
Learn more about equilibrium constant -
https://brainly.com/question/3159758
#SPJ4
The complete question is -
In a given experiment, 0.10 atm of each component in the following reaction is placed in a sealed container. In which direction will the reaction proceed?
2 NOBr (g) <==> 2 NO (g) + Br2 (g) where Kp = 60.6 at 100°C