When Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g), 98.8 kJ of energy are absorbed for each mole of Fe2O3(s) that reacts. Write a balanced thermochemical equation for the reaction with an energy term in kJ as part of the equation. Note that the answer box for the energy term is case sensitive. Use the SMALLEST INTEGER coefficients possible and put the energy term in the last box on the appropriate side of the equation. If a box is not needed, leave it blank.
Answers
Answer:
Explanation:
Fe₂O₃(s) + 3H₂(g) = 2Fe (s) + 3H₂O - 98.8 kJ .
one mole of ferric oxide reacts with 3 mole of hydrogen to give 2 mole of iron and 3 mole of water . 98.8 kJ of heat is absorbed .
Related Questions
AIDS is one disease caused by a virus infection. The virus attacks immune system cells known as T cells.
Based on your observations from the Gizmo, how would you explain the data shown on this graph?
Answers
Answer:
yes,its true
Explanation:
I just want to say first off
the immune cell known as T cell also known as white blood cells
Since the first main attack from the bodies response against a virus is deploying T cells to attack
CD4 cells are the basic T cells and HIV virus which is AIDS will start to "Eat itself inside" Ofc not they will use protein spikes to form their way in
they start to create copies of themselves inside the T cell itself and creating a new protein called Env to form their ways in ofc
They are able to bypass CD4 cells is because its a basic cellular receptor for T cells making them really deadly
They now will start to attack other white cells and deploying more and more of their virus cells
what does cost-effective mean? im kind of confused on cost-effective and time-effective things and i keep getting mixed up :(
Answers
Cost-effective definition: producing good results without costing a lot of money. E.g: that machine is very cost effective, it made 200 shirts with only a dollar.
Time effective definition: how well time was used. E.g that machine is very time-effective, it made 200 pants in one hour!
Would appreciate brainly <3
Number of electrons for all of these even the ones with a number by them ? Will give brainlist!!!!

Answers
Explanation:
s=2
p=6
d=10
f=14
pls mark me brainlist
Report the following measurement using the correct number of significant figures.
Answers
Based on the information, we can infer that the red line is about 3.9 cm.
How to identify the measure of the red line?To identify the measure of the red line we must look at the metric rule at the bottom. As we can see, this demarcates the centimeters and the millimeters. Based on the information, we can infer that the answer is 3.9 cm since the line extends to the line that delimits this value.
Based on the above, we can infer that the red line measures about 3.9 cm, taking the ruler as a reference.
Note: This question is incomplete. Here is the complete information:
Attached image
Learn more about measurements in: https://brainly.com/question/28913275
#SPJ1

C 4 H 15 O+O 2 -- CO 2 +H 2 O
Answers
Balance this reaction.
___CO + ___H2 → ___CH3OH
nevermind...
options for each blank: blank,2,3,4
Answers
Answer:
4 CO + 8 H2 -> 4 CH3OH
Hope that it works
A 25 L sample of oxygen gas (O2) has a mass of 48 grams and a pressure of 3.0 atm. What would be the temperature of the sample? Reminder: Use the equation PV=nRT, with the constant R = 0.0821 L atm/mol K.
A.
609 K
B.
305 K
C.
19.0 K
D.
1.60 x 10-2 K
Answers
The temperature of the oxygen gas sample is 609 K, which is approximately 336°C or 637°F. The answer is A.
We can use the ideal gas law equation, PV = nRT, to solve for the temperature of the oxygen gas sample.
First, we need to calculate the number of moles of oxygen gas present in the sample using its mass and molar mass:
n = m/M
where:
n = number of moles
m = mass (in grams)
M = molar mass (in g/mol)
The molar mass of oxygen gas (O2) is 32.00 g/mol.
n = 48 g / 32.00 g/mol = 1.50 mol
Next, we can rearrange the ideal gas law equation to solve for temperature (T):
T = (PV) / (nR)
where:
T = temperature (in Kelvin)
P = pressure (in atm)
V = volume (in liters)
n = number of moles
R = gas constant (0.0821 L atm/mol K)
Plugging in the given values, we get:
T = (3.0 atm x 25 L) / (1.50 mol x 0.0821 L atm/mol K)
T = 609 K
For more question on temperature click on
https://brainly.com/question/4735135
#SPJ11
The picture shows a model of a helium atom and a model of a lithium atom. Which of the following statements is true about the lithium and the helium atoms? A. Helium has more protons, neutrons, and electrons. B. There is no difference between helium and lithium. C. Lithium has more protons, neutrons, and electrons. D. Lithium is much smaller than helium.
Answers
Answer:
C. Lithium has more protons, neutrons, and electrons.
Answer: C. Lithium has more protons, neutrons, and electrons.
Explanation:
the perimeter of a isosceles is 48 meters. the upper base is 12 meters shorter than the lower base x.each leg is 12 meters shorter than the lower base x. each leg is 12 meters shorter than the lower base. write the equation of the perimeter in terms of x.
Answers
12/4=3
Which reactions performed in the experiment
involved chemical changes?
crushing calcium carbonate
mixing calcium carbonate and HCI
boiling water
heating copper (II) sulfate pentahydrate
separating iron filing and sulfur
mixing potassium iodide and lead nitrate
combining magnesium and HCI
burning the candle
DONE
Answers
Mixing calcium carbonate and HCl
Heating copper sulfate pentahydrate
Mixing potassium iodide and lead nitrate
Combining magnesium and HCl
Burning the candle.
The crushing of limestone is a bodily trade it no longer alters the chemical composition of the limestone. The heating of limestone is a chemical trade the limestone decomposes into two other materials, lime and carbon dioxide. both ended in gas formation. both ended in shade change.
Rust is not anything however Iron Oxide is a brand new substance shaped out of the response. The color of the floor of the iron additionally modifications. hence, rusting of iron is a chemical trade. Rusting is an instance of chemical exchange. A chemical property describes the ability of a substance to go through a particular chemical alternate. The chemical belonging of iron is that it is miles capable of combining with oxygen to form iron oxide, the chemical call of rust. like several steel carbonates, calcium carbonate reacts with acidic solutions to supply carbon dioxide gas.
Learn more about Experiment here:-https://brainly.com/question/17274244
#SPJ9
6. According to the graph above, which paper towel brand adsorbed the 5 points
most liquid?
Bounty
Brawny
Viva

Answers
61. Given the following information:
Ag2 CrO4(s)=2Agt (aq) + CrO4²- (aq)
Ag+ (aq) + e- Ag(s)
find the standard reduction potential at 25°C for the half-reaction
Ksp = 1 × 10-12
E = +0.799 V
Ag2 CrO4(s) + 2e¯ 2Ag(s) + CrO4²- (aq)
Answers
Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
To find the standard reduction potential at 25°C for the half-reaction Ag2CrO4(s) + 2e¯ → 2Ag(s) + CrO4²-(aq), we can use the Nernst equation, which relates the standard reduction potential (E°) to the equilibrium constant (K) and the reaction quotient (Q).
The Nernst equation is given as follows:
E = E° - (RT/nF) * ln(Q)
Given information:
Ksp = 1 × 10^(-12)
E = +0.799 V (standard reduction potential of Ag+ to Ag)
Since the reaction involves the dissolution of Ag2CrO4(s), the reaction quotient Q can be expressed as [Ag+]²/[CrO4²-].
Since the stoichiometry of the reaction is 2:1 for Ag2CrO4 to Ag+, we can say that [Ag+]² = Ksp.
Therefore, Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
Please note that without specific values for temperature (T) and the ideal gas constant (R), the exact standard reduction potential at 25°C cannot be determined.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
When 45.8 g of K2CO3 react with excess HCI, 46.3 g of KCl are formed. Calculate the theoretical and % yields of
KCI.
Answers
Answer:
Theoretical yield = 49.45g
Percentage yield= 93.6%


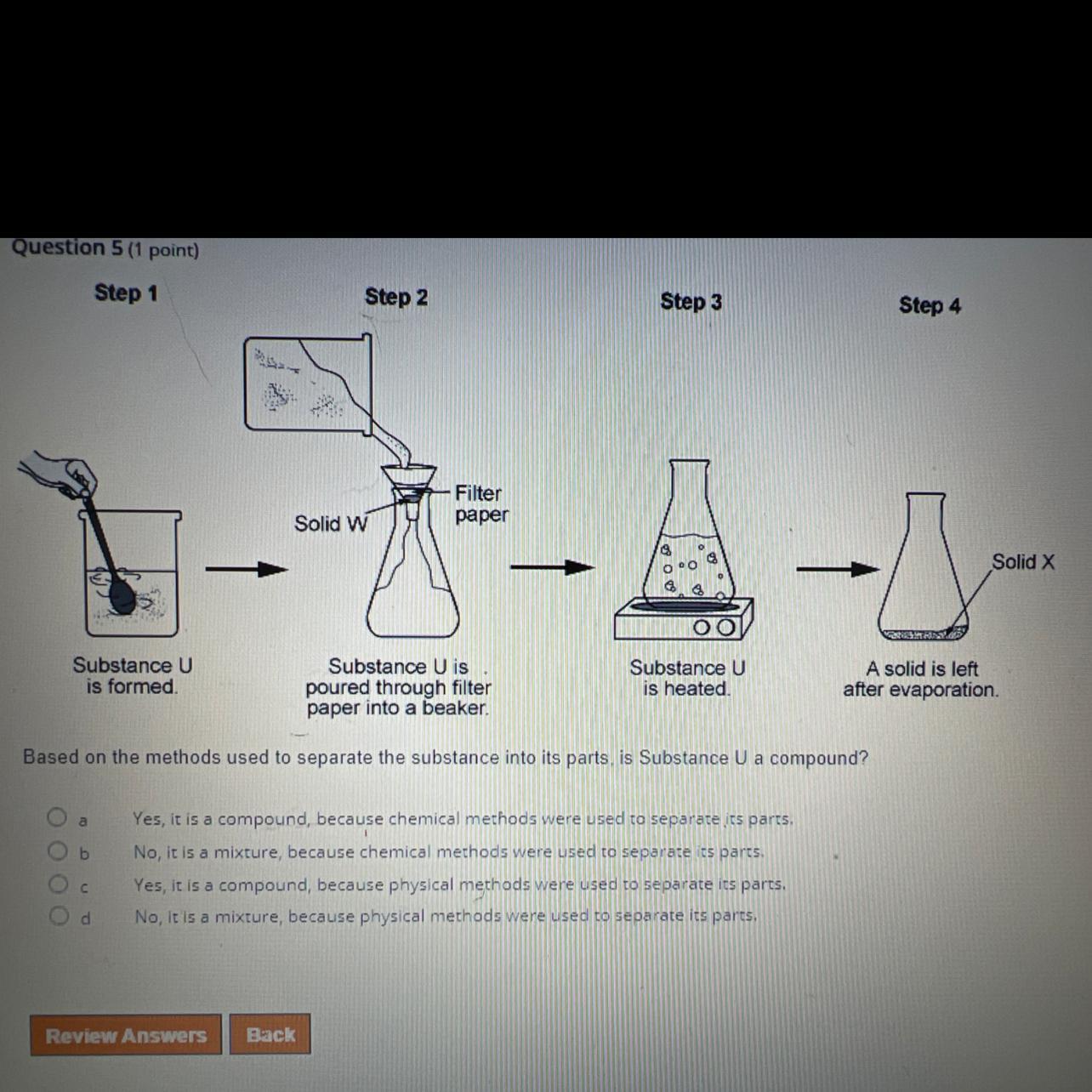
Based on the methods used to separate the substance into its parts is substance U a compound?
A. yes its a compound because chemical methods were used to separate its parts
B. no its a mixture because chemical methods were used to separate its parts
C. yes it is a compound because physical methods were used to separate its parts
D. no it is a mixture because physical methods were used to separate its parts
Answers
Based on the methods used to separate the substance into its parts is substance U is a mixture because physical methods were used to separate its parts; option D.
What are separation techniques?Separation techniques are techniques employed in the separation of mixtures of substances.
Mixtures are substance made up of two or more components physically joined together.
Considering the substance which is being separated, the separation techniques employed are physical separation techniques, hence the substance is a mixture.
In conclusion, physical separation techniques are employed in the separation of mixtures.
Learn more about separation techniques of mixtures at: https://brainly.com/question/4825542
#SPJ1
What is the balanced net ionic equation of mixed AgNO3 and MgCl2 solutions?Ag+(aq) + Cl2–(aq) → AgCl2(s)Mg2+(aq) + NO3–(aq) → Mg(NO3)2(s)Ag3+(aq) + 3Cl–(aq) → AgCl3(s)Ag+(aq) + Cl–(aq) → AgCl(s)
Answers
Answer
Ag⁺(aq) + Cl⁻ → AgCl(s)
Explanation
The balanced molecular equation of mixed AgNO3 and MgCl2 solutions is:
2AgNO₃(aq) + MgCl₂(aq) → 2AgCl(s) + Mg(NO₃)₂ (aq)
The balanced complete ionic equation for the reaction is:
2Ag⁺(aq) + 2NO₃⁻(aq) + Mg²⁺(aq) + 2Cl⁻ → 2AgCl(s) + Mg²⁺(aq) + 2NO₃⁻(aq)
NO₃⁻ ions and Mg²⁺ are spectators, remaining in the solution. Ag⁺ and Cl⁻ ions react to form a precipitate of AgCl according to this net ionic equation below.
The balanced net ionic equation is:
Ag⁺(aq) + Cl⁻ → AgCl(s)
What is the mass percent when 144 grams of NaCl is dissolved in 255 grams of water?
Answers
Answer:
36.09% approximately
Explanation:
I'm not good at English so I don't know if I get it right
Draw the structure of water. Is water considered polar or nonpolar? Why? Note: You can draw this on paper, take a picture, and paste the picture in your document.
Answers
Answer:
Water is polar
Explanation:
help me please :(
D & 1 is also a option

Answers
To make energy, a plant must get carbon, hydrogen, and oxygen atoms. What is the source of these atoms?
air and sunlight
water and air
air and soil
Water and soil
Answers
Answer:
Air and sunlight
Explanation:
To make energy, a plant must get carbon, hydrogen, and oxygen atoms. The source of these atoms are water and air.
What is Photosynthesis?The process in which green plants use sunlight to make their own food is called photosynthesis. Plants requires sunlight, chlorophyll, water, and carbon dioxide gas as raw materials. Chlorophyll is a substance that is present in leaves of the plant and makes plants greens. It consist of the metal Magnesium.
Photosynthesis also is done by other organisms besides green plants. These include prokaryotes like cyanobacteria, purple bacteria and green sulfur bacteria. These organisms exhibit photosynthesis just like green plants. The glucose is produced in them during photosynthesis and it is then used to fuel various cellular activities. The by-product of this physio-chemical process is oxygen.
Therefore, To make energy, a plant must get carbon, hydrogen, and oxygen atoms. The source of these atoms are water and air.
Learn more about photosynthesis, here:
https://brainly.com/question/29764662
#SPJ3
A burning match will burn more vigorously in pure oxygen than in air because _________ . Select one: a. oxygen is a catalyst for combustion b. nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion c. oxygen is a product of combustion d. nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Answers
Answer:
e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Explanation:
The reaction of a burning match is combustion. In this combustion, the organic components of the match (such as cellulose, C₆H₁₀O₅) react with oxygen, producing water and carbon dioxide:
C₆H₁₀O₅(s) + 6O₂(g) → 5H₂O(g) + 6CO₂(g)Seeing as oxygen is a reactant and not a catalyst nor product, and that nitrogen plays no part in the reaction, the only correct answer is option e.
Phosphorus pentachloride decomposes into Phosphorous trichloride and Chlorine gas. 0.670 moles of pure Phosphorus pentachloride is placed in a 4.00 L bottle. What are the resultingconcentrations?

Answers
Answer:
The resulting concentrations at equilibrium are:
[PCl5] = 0.168 M
[PCl3] = 0.0595 M
[Cl2] = 0.0595 M
Explanation:
The question requires us to calculate the concentrations of PCl3 and Cl2, given that 0.670 moles of PCl5 were placed in a 4.00 L container and the equilibrium constant of the following reaction is 0.0211 M:
\(PCl_{5(g)}\rightleftarrows PCl_{3(g)}+Cl_{2(g)}\)We can solve this problem using the equilibrium constant expression, considering that the concentration of PCl3 and Cl2 at the equilibrium will be the same (note that they present the same stoichiometric coefficient).
We can write the expression for the equilibrium constant as:
\(K=\frac{[PCl_3\rbrack\times[Cl_2\rbrack}{[PCl_5\rbrack}\)Considering that [PCl3] = [Cl2] = x, we can rearrange this expression to calculate x:
\(x^2=K\times[PCl_5\rbrack\rightarrow x=\sqrt[x]{K\times[PCl_5\rbrack}\)The concentration of PCl5 can be obtained from the number of moles given (0.670 moles) and the volume of the container (4.00L):
\([PCl_5\rbrack=\frac{0.670mol}{4.00L}=0.168mol/L=0.168M\)Now, applying the values of K (0.0211M) and [PCl5] (0.168M) to the expression we wrote above, we'll have:
\(\begin{gathered} \begin{equation*} x=\sqrt[x]{K\times[PCl_5\rbrack} \end{equation*} \\ \\ x=\sqrt[2]{0.0211M\times0.168M}=0.0595M \end{gathered}\)Therefore, the resulting concentrations at equilibrium are:
[PCl5] = 0.168 M
[PCl3] = 0.0595 M
[Cl2] = 0.0595 M
Find the difference between the numbers. (2.260×104)−(8.098×103)
Answers
The difference between the numbers,
(2.260×104)−(8.098×103) = 598.539.
What is subtraction?Subtraction is a mathematic operation. Which is used to remove terms or objects in an expression.
Given:
Two numbers,
(2.260×104) and (8.098×103).
First, we simplify the numbers,
(2.260×104) = 235.04
And (8.098×103) = 833.579.
The difference between the numbers,
(2.260×104)−(8.098×103) = 833.579 - 235.04.
(2.260×104)−(8.098×103) = 598.539
Therefore, then value is 598.539.
To learn more about the subtraction;
https://brainly.com/question/2346316
#SPJ1
For the reaction N2O4(g) 2 NO2(g), Kc = 0.21 at 100 C. At a point during the reaction, [N2O4]=0.12 M and [NO2]=0.55M. Is the reaction at equilibrium? If not, in which direction is it progressing?
Answers
The equilibrium constant shows the extent to which reactants are converted into products.
In this case, we must find the reaction quotient as follows;
Qc = [0.55]^2/[0.12] = 2.5
Since the Qc > Kc, it means that the reverse direction is favored in this reaction.
Learn more about Kc:https://brainly.com/question/15856317
#SPJ1
Methane (CH_4) gas is used for heating in many applications. It is gas used to fuel the Bunsen burners in this classroom. The chemical equation for the combustion of methane is given. Answer the following questions.
CH_4 + 2O_2 --> CO_2 + 2H_2O
1.) Will the ∆H for the combustion of methane be +890 kJ/mol or -890kJ/mol? Explain.
2.) Is energy a reactant or a product in this reaction?
Answers
sign of ∆H :-
+ve means endothermic reaction -ve means exothermic reactionWe know
when methane burns in presence of oxygen heat is released as a form of energy so the reaction is exothermic.∆H must be -ve
#2
Product as energy is released so it will be in right sideWhy is there an octet rule in writing Lewis structures?
Answers
Answer:
Before we can sketch the Lewis structures of molecules, we must first understand the octet rule. The octet rule asserts that when atoms combine to create compounds, electrons are gained, lost, or shared among them, resulting in a stable electron configuration defined by eight valence electrons as a result. These rules are used in conjunction with the main-group components of the second period.
Explanation:
Hope it helps:)
What does relative humidity measure?
• the amount of water in cirrus clouds
• the amount of water hat has evaporated into the air
• the amount of frozen water that has condensed from the air
• the amount of water valor in the air compared to the amount it can hold
Thank you so much! have a nice day
Answers
Answer:
the amount of water vapor in the air compared to the amount it can hold
Explanation:
By definition, relative humidity is equal to the partial pressure of water divided by the total amount of water that the air can hold at that temperature. The last one basically restates the definition. (Partial pressure is the number of particles of a substance divided by the total number of particles.)
(Have a nice day too! Don't hesitate to ask any questions)
Calculate the concentration (in molarity) of an NaH solution if 25.0 mL of the solution is needed to neutralize 19.7 mL of a 0.463 M HCI solution.
Answers
Answer:
0.11 m
Explanation:
H Ci + NaOH H2 O+ NaC1
by titration
0.0025 L of 0.13 mol Na OH = 0.00325 Mol NaOH
L
0.00325 Mol NaOH 1 Mol Hcl/I Mol NaOH = 0.00325 Mol hcl
now 0.00325 mol Hcl/ 0.030 L = 0.0108 M hcl
A compound is made by carbon, hydrogena and nitrogen and 1 g of this compound contains 91.5 mg of hydrogen and 424 mg of nitrogen. What is the molecular formula given that the molar mass is about 65 g/mol? (write the formula with elements in this sequence HNO)
Answers
Answer:
C₄H₃N
Explanation:
To solve this question we must find the moles of each atom in order to find the empirical formula (Simplest ratio of atoms present in a molecule). With empirical formula and the molar mass of the compound we can find the molecular formula as follows:
Moles H:
91.5mg H * (1mmol / 1mg) =
91.5mmol H
Moles N:
424mg N * (1mmol / 14mg) =
30.286mmol N
The ratio of moles regard to moles N (The lower number of moles is:
H = 91.5mmolH / 30.286mmolN = 3.0
N = 30.286mmolN / 30.286mmolN = 1
empirical formula = CₓH₃N
The molar mass of this formula is:
12*x + 1*3 + 1*14 = 17g/mol
Solving for X:
12X + 17g/mol = 65g/mol
12X = 48g/mol
X = 4
There are 4 atoms of carbon and molecular formula is:
C₄H₃N1 In general, how many major glands are found in human body?
A. Eight
B. Ten
C! Thirty-two,
D. Forty six
Answers
Answer:
A. Eight
Explanation:
Although there are eight major endocrine glands scattered throughout the body, they are still considered to be one system because they have similar functions, similar mechanisms of influence, and many important interrelationships.
I need help. Thanks if you helped.


Answers
Answer:
Hi! The answer to the first question is A. Nina hears the sound of her drum. This occurs last because all the other events must occur first in order for Nina to hear the drum. She must hit it, the surface of the drum must vibrate, and the sound waves must travel through the air. Only then will she hear the drum.
The answer to the second question is A. Hertz. The number of vibrations per second, or frequency, is measured in hertz (Hz).
Have a great day! - Mani :)