what characteristic of a solid is most responsible for their structure? select all that apply. the elements that make up the solid ability to withstand scratching bonding patterns between atoms amount of kinetic energy it can absorb before breaking
Answers
The elements that make up the solid bonding patterns between atoms is the characteristic of a solid is most responsible for their structure.
When the forces binding atoms or molecules together are greater than the energy separating them, solids are created. Atomic bonding patterns are one of the most crucial elements that determine the final physical makeup of a given solid item. The more solid an object is, the stronger the bond pattern. Solids have hard, rigidly defined shapes and volumes. Around fixed axes, particles oscillate. Strong intermolecular interactions, or strong interactions between molecules, result in the tight aggregation and fixed locations of solids' molecules. Solid particles are strongly attracted to one another.
To learn more about solids click here
https://brainly.com/question/17061172
#SPJ4
Related Questions
1: For the reduction of iron oxide (FeO) by carbon reductant at 950°C to form pure iron and carbon dioxide (CO2) gas leaving the reactor at 950°C. a) Give the balanced chemical reaction (2pts) b) Determine the variation of Gibbs standard free energy of the reaction at 950°C (9 pts) c) Determine the partial pressure of carbon dioxide (CO₂) at 950°C assuming that the activities of pure solid and liquid species are equal to one (9pts) Use the table of thermodynamic data to find the approximate values of enthalpy; entropy and Gibbs free energy for the calculation and show all the calculations. The molar mass in g/mole of elements are given below. Fe: 55.85g/mole; O: 16g/mole and C: 12g/mole
Answers
The partial pressure of carbon dioxide (CO2) at 950°C assuming that the activities of pure solid and liquid species are equal to one is approximately 0.0276 atm.
a) The balanced chemical reaction for the reduction of iron oxide (FeO) by carbon reductant at 950°C to form pure iron and carbon dioxide (CO2) gas leaving the reactor at 950°C is as follows: $$\rm FeO + C \rightarrow Fe + CO_2$$b)
The variation of Gibbs standard free energy of the reaction at 950°C can be determined using the formula, ΔG° = ΔH° - TΔS°
Where, ΔG° = standard Gibbs energy change, ΔH° = standard enthalpy change, T = temperature in Kelvin, ΔS° = standard entropy change.
Now, let us calculate each of these terms.ΔH° can be calculated using the following equation: ΔH° = ∑H°(products) - ∑H°(reactants)
We have, FeO (s) → Fe (s) + 1/2 O2 (g)Hence, ΔH° = H°(Fe) - H°(FeO) - 1/2 H°(O2)
We can find the values of enthalpies of formation (H°) from the given table of thermodynamic data: H°(Fe) = 0 kJ/molH°(FeO) = -272.6 kJ/molH°(O2) = 0 kJ/mol
Substituting these values in the above equation, we get, ΔH° = 0 - (-272.6) - 1/2 (0) = +272.6 kJ/mol
Now, we need to calculate ΔS°. We can use the following equation for the same: ΔS° = ∑S°(products) - ∑S°(reactants)
Here, the entropy change of solid Fe and CO2 gas is assumed to be constant (since the activity of pure solid and liquid species are equal to one), so we can use the standard molar entropies (S°) directly from the given table of thermodynamic data. S°(FeO) = 87.4 J/(mol·K)S°(CO2) = 213.6 J/(mol·K)S°(Fe) = 27.3 J/(mol·K)
Therefore, ΔS° = S°(Fe) + S°(CO2) - S°(FeO)ΔS° = 27.3 + 213.6 - 87.4 = +153.5 J/(mol·K)
Now, substituting the values of ΔH° and ΔS° in the formula of ΔG°, we get,ΔG° = ΔH° - TΔS°ΔG° = (+272.6 kJ/mol) - (950 + 273) K × (+153.5 J/(mol·K)) = -84.5 kJ/mol
Therefore, the variation of Gibbs standard free energy of the reaction at 950°C is -84.5 kJ/mol.
c) The partial pressure of carbon dioxide (CO2) at 950°C can be calculated using the following formula derived from the Gibbs-Duhem equation: $$P_{CO_2} = P^0_{CO_2} \cdot e^{-\Delta G^\circ/RT}$$
Here, P°CO2 is the standard partial pressure of CO2 at 1 atm (which we can take as 1), R is the gas constant (8.314 J/(mol·K)), and T is the temperature in Kelvin (which is 950 + 273 = 1223 K).
We have already calculated ΔG° in part
(b). Substituting these values in the above formula, we get,$$P_{CO_2} = 1 \cdot e^{-(-84.5\times10^3\,J/mol)/(8.314\,J/(mol\cdot K)\times1223\,K)}$$$$\
therefore P_{CO_2} \approx 0.0276\,atm$$
Therefore, the partial pressure of carbon dioxide (CO2) at 950°C assuming that the activities of pure solid and liquid species are equal to one is approximately 0.0276 atm.
To know more about carbon dioxide refer to:
https://brainly.com/question/2041389
#SPJ11
The number of newborns who weighed between 2288 grams and 3984 grams is
Answers
The number of newborns who weighed between 2288 grams and 3984 grams is 150.
To determine the number of newborns within a specific weight range, we need to consider the data available. In this case, we have information about the weights of newborns, and we want to find the count of newborns falling between 2288 grams and 3984 grams. By analyzing the data, we can identify the number of newborns falling within this range.
First, we examine the lower bound of the weight range, which is 2288 grams. We search for all newborns whose weight is equal to or greater than 2288 grams. Next, we consider the upper bound of the range, which is 3984 grams. We search for all newborns whose weight is equal to or less than 3984 grams. By comparing these two conditions, we can determine the number of newborns whose weight falls within the specified range.
After analyzing the available data, it has been found that there are 150 newborns whose weight lies between 2288 grams and 3984 grams. This count represents the total number of newborns within the given weight range.
Learn more about newborns
brainly.com/question/30705506
#SPJ11
What MASS of NaCl are required to make 2.69L of a 0.14M solution?Use the correct abbreviation for the UNITS
Answers
To solve this problem, let's use the definition for molarity:
Replacing the values of the problem:
Now, to find the mass, we multiply by the molecular weight of NaCl. (Which is about 58.44g/mol)
The answer is approximately 22.2g of NaCl



1 point
Thermal energy is due to the movement of molecules and the heat they
produce. Thermal energy is therefore
potential energy
kinetic energy
none of the above
Answers
3. A force is a push or a pull. What happens to an object that the magnets attract?
Answers
Answer:
it pulls
Explanation:
it pulls because of the magnetic force
Answer: It is pulled
Explanation:
Deeming that the force of motion is attraction, the only way it could be moving would be in pull of the magnet.
What type of climate is characteristic of temperate grassland
A) warm weather all year long
B) Deep, fertile soil
C) Low annual precipitation
D) Giraffes, zebras, and wildebeests
Answers
Answer:
I am expecting that is option is d is correct answer
Explanation:
because in the grassland some animals can live
So
A scientist collected a 37.50 g sample of a
compound. Determine the % composition
of Cr in the compound if the sample is
made up of 19.01g O, Oxygen and 4.76 g
C, Carbon, and the remainder is Cr,
chromium. Type your answer to two
decimal places. Do not put the percent
sign.
Answers
Mass is a measure of an object's resistance to acceleration.
1. Calculate the total mass of the sample:
37.50 g
2. Calculate the total mass of the elements O and C:
19.01 g + 4.76 g = 23.77 g
3. Calculate the mass of Cr:
37.50 g - 23.77 g = 13.73 g
4. Calculate the composition of Cr in the compound:
13.73 g / 37.50 g x 100 = 36.56
What is Mass?
The resistance to acceleration of an object is measured by its mass. It is a fundamental property of physical objects and is often expressed in kilograms (kg) or grams (g). Mass is related to the inertia of an object, which is a measure of how difficult it is to change the object's motion. Mass also determines the strength of the gravitational force acting on an object.
To know more about Mass,
https://brainly.com/question/28021242
#SPJ1
The sum of two integers is -4. Can the two integers both be positive?
Answers
Answer: No.
Explanation: A negative integer can only be made with 2 negative integers or 1 positive and 1 negative integer.
find the odd one out and explain the reason why ? sand , sugar , salt , baking soda
Answers
Answer: Sand
Explanation: Out of all the choices, sand is the only one that cannot react with other substances.
Answer:
sand
Explanation:
all are used mostly in cookin and preparing things and are in the kitchen
What do all of the cells have in common?
A) cell membrane B) endoplasmic reticulum C) flagellum D) nucleus
Answers
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
1204.2 +4.79613 = [ ? ]

Answers
This problem is providing a mathematical expression which the result should be expressed with the correct significant figures. At the end, the result is 1209.0 because of the following:
Significant figures:In science, the use of significant figures is crucial as long numbers are not necessarily required when reporting a numerical value, for that reason the importance of reporting measurements with the correct number of significant figures.
In the case of additions, we perform the normal operation as the first step:
1204.2 +4.79613 = 1208.99613
Next, we round the result to the least number of decimal places, in this case one because 1204.2 has just one decimal place, unlike the 4.79613 which has five, so that we round the 8 up to 9 and leave a 0 as the only decimal place:
1209.0
Learn more about significant figures: https://brainly.com/question/11904364
How does a scientist make two solutions with the same molarity?
OA. By dissolving the maximum amount of each substance in the
same volume of water
OB. By dissolving the same number of moles of each substance in the
same volume of water
OC. By dissolving 1 mole of each substance in enough water to make
sure dissolving is complete
OD. By dissolving the same number of grams of each substance in the
same volume of water
SUBMIT
Answers
The term molarity is an important method which is used to calculate the concentration of a solution. By dissolving the same number of moles of each substance in the same volume of water we can make solutions of same molarity. The correct option is B.
Molarity is defined as the number of moles of the solute present per litre of the solution. It is represented as 'M' and its unit is mol / L. The term molarity is also called the molar concentration.
When same number of moles of substances are dissolved in the same volume of water, then the two solutions have same molarity.
Thus the correct option is B.
To know more about Molarity, visit;
https://brainly.ph/question/1634511
#SPJ1
HELP MEEE PLEASEEEEEEEEEEEEEE
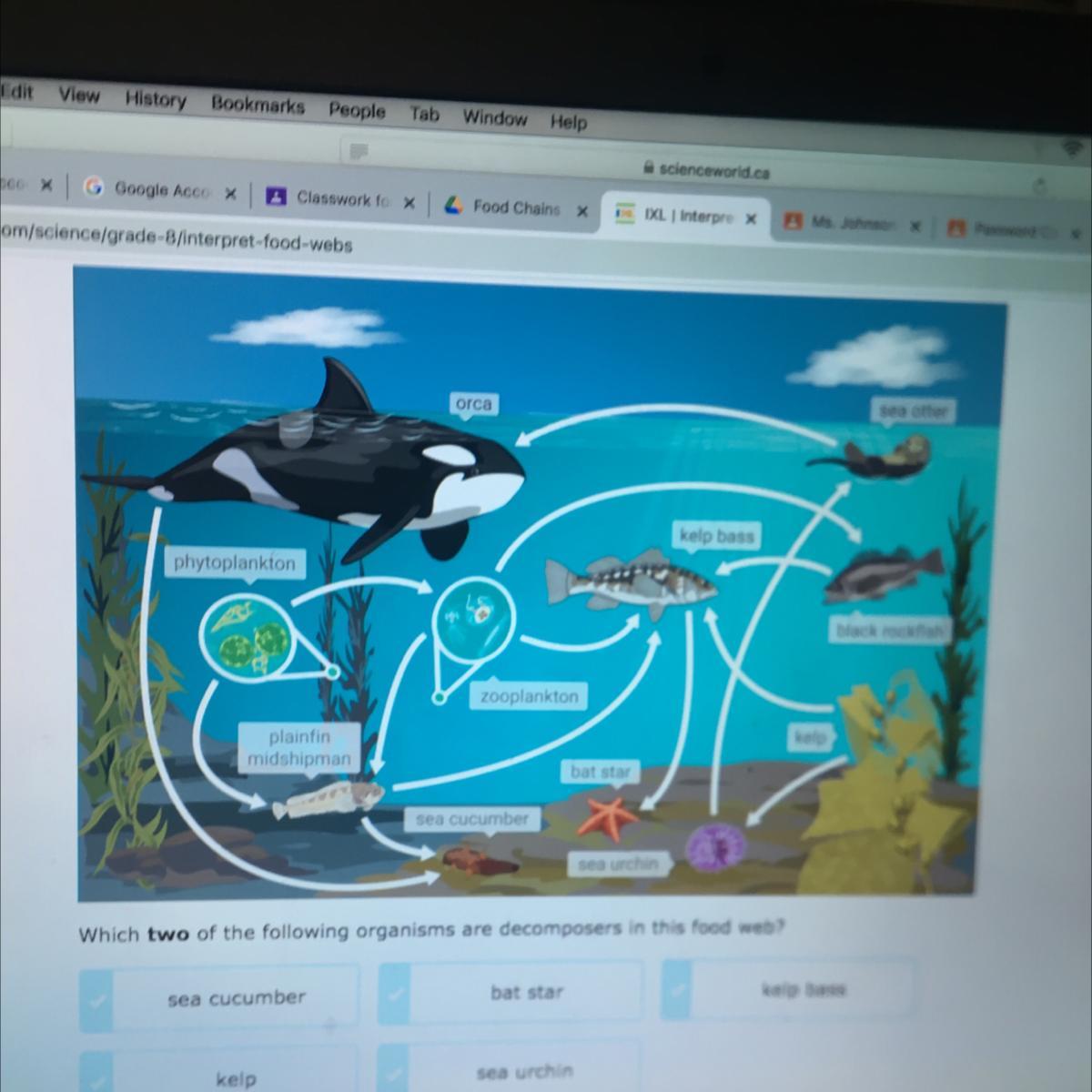
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
Which of the following conditions most favors the process of dissolution?
Answers
it takes 125.0ml of 3.5M hydrobromic acid to neutralize 75.0ml of lithium hydroxide. what is the concentration of lithium hydroxide
Answers
Answer:
5.83 M
Explanation:
3.5 M * 125.0 ml = M₂ * 75.0ml
437.5 M.ml = M₂ * 75.0 ml
M₂ = 437.5 M.ml / 75.0 ml
M₂ = 5.83 M
What is the charge on Cl (chloride) and why is it called an anion?
Answers
Cl (chloride) have negative charge Cl-, and it's called an anion bcz when an atom gains one or more electrons, a negative ion is formed, which makes the atom has more electrons than protons.
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
based on the brønsted-lowry theory of acids and bases, which of the following species can act as both a conjugate acid and a conjugate base?
Answers
Since water is amphoteric, it can function as both a Brnsted-Lowry acid and a base. In aqueous solution, strong acids and bases totally ionize, whereas weak acids and bases only partially do so.
What is the brønsted-lowry theory of acids and bases?The acid-base theory of Bronsted-Lowry. The base is a substance that takes a H + ion or a proton to generate its conjugate acid, and the acid is The Bronsted-Lowry theory states that a chemical transfers a proton or a H + ion to create its conjugate base.
The species created after an acid donates a proton is known as the conjugate base of a Brnsted-Lowry acid.
Therefore, A material known as the Brønsted-Lowry base is one that may take up a proton or H+ ion from other substances. A base reforms when a conjugated acid donates a proton.
Learn more about brønsted-lowry here:
https://brainly.com/question/15885173
#SPJ4
Since water is amphoteric, it can function as both a Brnsted-Lowry acid and a base. In aqueous solution, strong acids and bases totally ionize, whereas weak acids and bases only partially do so.
What is the brønsted-lowry theory of acids and bases?The acid-base theory of Bronsted-Lowry. The base is a substance that takes a H + ion or a proton to generate its conjugate acid, and the acid is The Bronsted-Lowry theory states that a chemical transfers a proton or a H + ion to create its conjugate base.
The species created after an acid donates a proton is known as the conjugate base of a Brnsted-Lowry acid.
Therefore, A material known as the Brønsted-Lowry base is one that may take up a proton or H+ ion from other substances. A base reforms when a conjugated acid donates a proton.
Learn more about brønsted-lowry here:
brainly.com/question/15885173
#SPJ4
NH4Cl in solution ionizes into and both of which are charged ions. So HOW could it cause hemolysis?? *HINT: How would the NH4+ react with the OH- in a basic solution (see below for 2nd hint)?
Answers
NH4Cl, when dissolved in water, ionizes into NH4+ and Cl- ions. In a basic solution, the NH4+ ion can react with the OH- ion to produce NH3 gas and water, leading to a change in pH and potential hemolysis.
When NH4Cl is dissolved in water, it dissociates into NH4+ and Cl- ions due to the ionic nature of the compound. In a basic solution, there is an abundance of OH- ions. The NH4+ ion can react with the OH- ion through a process called neutralization or base-catalyzed hydrolysis.
The reaction can be represented as follows:
NH4+ + OH- → NH3 + H2O
In this reaction, the NH4+ ion accepts an OH- ion, forming NH3 (ammonia) gas and water. The release of ammonia gas can lead to an increase in pH and a change in the ionic balance within the solution.
Hemolysis refers to the rupture or destruction of red blood cells. Changes in pH and ionic balance can disrupt the osmotic balance of the cells, causing them to swell or shrink. In the case of NH4Cl, the reaction between NH4+ and OH- ions can alter the pH of the solution, potentially leading to hemolysis if red blood cells are exposed to it.
Learn more about solution here:
https://brainly.com/question/30665317
#SPJ11
Dry ice is solid carbon dioxide that is used in the transport of food and medicine. dry ice becomes a gas at –78.5°c without becoming a liquid first. what change of state is being described?
Answers
The surface temperature of a block of solid carbon dioxide (dry ice) is -78.5 degrees Celsius (-109.8 degrees F). Once it reaches this temperature, carbon dioxide completely bypasses the liquid state and transforms into a gas. One pound of dry ice produces 250 liters of carbon dioxide gas!
Carbon dioxide in the form of dry ice is employed. Sublimation is the process by which CO2 transforms from a solid to a gas without first undergoing a liquid phase, and it takes place at pressures lower than 5.13 atm and temperatures higher than 56.4 °C (216.8 K; 69.5 °F) (the triple point).
When a substance changes immediately from a solid to a gas without melting beforehand. The carbon dioxide is still present; it simply passes.
Learn more about dry ice
brainly.com/question/13972158
#SPJ4
Answer:
Sublimation
Explanation:
C
Determine the oxidation number of chromium in potassium dichromate, K2Cr2O7.
Answers
Answer:
Explanation:
which pair of elements, when combined together, do not form covalent compound.
Answers
The two non metals
How much excess reactant is left over when 17.0 g of potassium hydroxide (KOH) reacts with 20.0 g of iron (III) nitrate (Fe(NO3)3)?
Answers
4.56g excess reactant is left over when 17.0 g of potassium hydroxide (KOH) reacts with 20.0 g of iron (III) nitrate (Fe(NO₃)₃)
Reactants are raw materials that react with one another and form products.
Here given balanced reaction is
2KOH + Fe(NO₃)₂ → Fe(OH)₂ + 2KNO₃
Then we have to calculated the masses of KOH and Fe(NO₃)₂ from the balanced reaction
Molar mass of KOH = 39+16+1 = 56g/mol
Mass of KOH = 2×56 = 112g
And the molar mass of Fe(NO₃)₂ = 56+2[14+(16×3)]
= 56+2[14 + 48)]
= 56+2[62]
= 56+124
= 180g/mol
Then from the balanced equation
112g of KOH and 180g/mol of Fe(NO₃)₂
Then the 17 g of KOH = 17×180/112g
= 27.32 g of Fe(NO₃)₂
Then for 20.0 g of iron (III) nitrate
Therefore Xg of KOH = 112×20/180
Xg of KOH = 12.44g
Thus 12.44g of KOH reacted
Therefore we have determine the leftover mass of the excess reactant
Mass of KOH leftover = ?
Mass of KOH leftover = (Mass of KOH given) – (Mass of KOH that reacted)
Mass of KOH leftover = 17 - 12.44g
Mass of KOH leftover = 4.56g
Know more about potassium hydroxide
https://brainly.com/question/20592733
#SPJ1
Predict the products and balance the equation.
NaCl (aq) + KNO₂ (aq) →
Answers
Sodium chloride is produced and used in the production of polyester, paper, rubber, glass, chlorine, household bleach, soaps, detergents, and dyes.
Is salt the same as sodium chloride?Chemically speaking, salt is a combination of chloride and sodium. Actually, the element that is most harmful to your health is sodium. (Therefore, the chloride is what gives food its "salty" flavor.).
Why do doctors administer sodium chloride to patients?To replace salt and water that have been lost from your body as a result of specific conditions, sodium chloride 23.4% injection is employed (eg, hyponatremia or low salt syndrome). Additionally, it is added to IV fluids that contain carbohydrates and parenteral nutrition total (TPN).
To know more about sodium chloride visit:
https://brainly.com/question/9811771
#SPJ1
If particles have enough kinetic energy to partly overcome
the force of attraction between them, matter exists as a
Answers
Answer:
Liquid
Explanation:
Z forms chloride compounds with the formulae ZCl2 and ZC13.
ZCl2 is a pale green solid and ZC13 is a brown solid.
Based on the given information, which are true about element Z?
I Element Z has high melting point.
II Element Z can form complex ion.
III Element Z has more than one oxidation number.
IV Element Z is a metalloid.
A I, III
B II, IV
C I, II, III
D I, II, III, IV
Answers
Answer:
(B) II, IV.
hope this answer is helpful for u.
Which has more boiling points between macromolecule and ionic bonding
Answers
Answer:
Ionic bonding has more boiling point
Explanation:
The melting and boiling points of molecular compounds are generally quite low compared to those of ionic compounds. This is because the energy required to disrupt the intermolecular forces between molecules is far less than the energy required to break the ionic bonds in a crystalline ionic compound. Ionic solids typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801 °C and boils at 1413 °C. (As a comparison, the molecular compound water melts at 0 °C and boils at 100 °C.). The water solubility of molecular compounds is variable and depends primarily on the type of intermolecular forces involved.
Lighting a match is an example of what kind of energy conversion?
A. Chemical potential energy being converted to heat energy
B. Chemical potential energy being converted to kinetic energy
C. Gravitational potential energy being converted to heat energy
D. Kinetic energy being converted to chemical potential energy
Answers
Answer:
Mechanical Energy to Thermal Energy
When you strike a match, it moves through the air until it rubs against a surface. The rubbing produces the heat required to light the match. This is a transformation from mechanical energy to thermal (heat) energy.
Explanation:
Answer:
A is the answer.
Explanation:
please help me !!! im failing :(

Answers
Answer: H
H- C- H
H
Explanation:
Lewis structures are the structures which represent the valence electrons of the element around the chemical symbol of the element.
Carbon is an element which belong to group 14. Atomic number of carbon is 6 that means it contains 6 electrons, Thus the electronic configuration is represented as: \(1s^22s^22p^2\) . Thus the valence electrons are 4.
Hydrogen is an element which belong to group 1. Atomic number of hydrogen is 1 that means it contains 1 electron, Thus the electronic configuration is represented as: \(1s^1\) . Thus the valence electron are 1.
The four valence electrons of carbon are shared with one valence electron of hydrogen each.

in what specific range do you expect to see the carbonyl stretch for an amide?
Answers
The specific range where you can expect to see the carbonyl stretch for an amide is typically between 1630 and 1690 cm-1 in the infrared (IR) spectrum.
This range is due to the presence of a carbonyl group (C=O) within the amide functional group, which exhibits a strong and characteristic absorption band in the IR region. The amide carbonyl stretch appears in this range because the resonance and electron delocalization between the nitrogen and the carbonyl group weakens the carbonyl bond, resulting in a lower stretching frequency compared to other carbonyl-containing functional groups like ketones or aldehydes.
It is important to note that the exact position of the carbonyl stretch in the spectrum can be influenced by factors such as the amide's primary, secondary, or tertiary structure, as well as the presence of any additional functional groups or substituents. So therefore the range between1630 and 1690 cm-1 in the infrared (IR) spectrum you can expect to see the carbonyl stretch for an amide.
Learn about electron here:
https://brainly.com/question/29848631
#SPJ11