The ΔHvap of a certain compound is 14.17 kJ·mol−1 and its Δvap is 93.89 J·mol−1·K−1.
What is the boiling point of this compound?
Answers
The boiling point of a certain compound can be calculated using the Clausius-Clapeyron equation. This equation relates the boiling point of the substance to its heat of vaporization and the vapor pressure at a given temperature.
In the equation, R is the ideal gas constant, T is the boiling point, ΔHvap is the heat of vaporization, and Δvap is the molar volume of vapor.Explanation:According to the Clausius-Clapeyron equation, we have:ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)where:P1 is the vapor pressure at the boiling point of the compound,P2 is the vapor pressure at the temperature T2,ΔHvap is the heat of vaporization of the compound,R is the gas constant,T1 is the boiling point of the compound, andT2 is the temperature at which the vapor pressure is P2.
Rearranging this equation, we get:T2 = ΔHvap / R * (1/T1 - ln(P2/P1))Now, let's substitute the given values:ΔHvap = 14.17 kJ·mol−1 = 14,170 J·mol−1R = 8.314 J·mol−1·K−1Δvap = 93.89 J·mol−1·K−1P1 = 1 atm = 101.325 kPaP2 = 0.1 atm = 10.1325 kPaPlugging these values into the Clausius-Clapeyron equation:ln(10.1325/101.325) = -14,170/(8.314*T2)(-2.303) = -14,170/(8.314*T2)T2 = 463.3 KSo, the boiling point of the compound is 463.3 K.
To know more about boiling point visit:
https://brainly.com/question/2153588
#SPJ11
Related Questions
A sample of a compound has a total mass of 200.0 g. Phosphorus comprises87.28 g of the sample while oxygen comprises 112.72 g.a. Find the percent mass of phosphorus and oxygen in the sample.
Answers
To find the mass percent composition of an element, divide the mass contribution of the element by the total mass then multiply by 100.
The total mass = 200.0 g
Phosphorus mass = 87.28 g
Oxygen mass = 112.72 g
%composition of P:
=> (87.28 g/200.0g)*100
=> 43.64 %
%composition of O:
=> (112.72g/200.0 g)*100
=> 56.36 %
describe what happens when he touches the doorknob. observe which direction the spark travels when john's finger comes close to the metal doorknob
Answers
Static charge is developed within a body temporarily with an induced polarization by rubbing on his leg . Electrons are exchanged from his hand to the door when touched .
What is static electricity?When two materials rub against each other in which one to be good conductor, they will attract by the induced polarization. Rubbing causes the free electrons in the condutor to be transferred towards the surface in contact.
This will cause a deformation that, the random charges in the material get polarized where the electrons from one surface will repel to the opposite pol and positive charges will align in a pole close to the charged body.
This charge separation causes the positive pole of the metal gets attracted into the hand. Hence, the electrons from the hand move to the positive pole of metal and the spark generate in the opposite direction.
To find more on static electricity, refer here:
http://brainly.com/question/12791045
#SPJ1
What MASS of NaCl are required to make 2.69L of a 0.14M solution?Use the correct abbreviation for the UNITS
Answers
To solve this problem, let's use the definition for molarity:
Replacing the values of the problem:
Now, to find the mass, we multiply by the molecular weight of NaCl. (Which is about 58.44g/mol)
The answer is approximately 22.2g of NaCl



For 2 hours, Leah was driving east at one-half of her car's top speed. Her car can go at a maximum speed of 180 kilometers per hour. In this time, how far did Leah drive?
Answers
Answer:
180 kilometers in 2 hours.
Explanation:
To find the distance Leah drove, we need to first determine her average speed. Since Leah was driving east at one-half of her car's top speed, her average speed was 180/2 = 90 kilometers per hour.
Next, we can use this speed to determine the distance she traveled. Since Leah was driving for 2 hours at an average speed of 90 kilometers per hour, she traveled 2 * 90 = 180 kilometers.
Therefore, Leah drove 180 kilometers in 2 hours while driving east at one-half of her car's top speed.
In the following equation, ______ is being oxidized and ______ is being reduced.
CO3 2- + 2H+ → CO2 + H2O
A. None of these
B. carbon, oxygen
C. carbon, hydrogen
D. hydrogen, carbon
Answers
\(oxidation \: number \: of \: oxygen = \\ before \: rxn = - 2 \\ after \: rxn = - 2\)
\(oxidation \: number \: of \: hydrogen = \\ before \: rxn = + 1 \\ after \: rxn = \\ 2x - 2 = 0 \\ x = + 1\)
\(oxidation \: number \: of \: carbon = \\ before \: rxn = \\ x - 6 = - 2 \\ x = 4 \\ after \: rxn = \\ x - 4 = 0 \\ x = 4\)
Option A\(oxidation \: numbers \: remain \: constant \\ so \: none \:a re \: undergoing \: oxidation \: \\ nor \: reduction \: \)
Answer:
D
Explanation:
I believe that is the answer
suppose you are working with a novel molecule extracted from an herb to see if this molecule is an agonist for dopamine. this means that
Answers
Dopamine is a neurotransmitter in the brain involved in various physiological functions, including movement, reward, motivation, and pleasure.
As a researcher working with the novel molecule, your goal would be to investigate whether it exhibits agonistic activity towards dopamine receptors. This involves studying its ability to bind to dopamine receptors and initiate the associated signaling pathways. To determine if the molecule is a dopamine agonist, you would typically conduct experiments using in vitro and/or in vivo models. In vitro experiments involve working with isolated components, such as cell cultures or purified receptor proteins, while in vivo experiments involve studying the molecule's effects in whole organisms.
Learn more about Dopamine here:
https://brainly.com/question/31812698
#SPJ11
a dat is performed. the following results are obtained: polyspecific ahg - 1 saline control - 0 anti-igg - 1 anti-c3bc3d - 0 what are they coated? group of answer choices no answer is correct complement igm igg
Answers
DAT stands for direct antiglobulin test which is used to determine whether red blood cells (RBCs) have been coated in vivo with immunoglobulin, complement, or both.
A direct antiglobulin test (DAT) is used to determine whether red blood cells (RBCs) are coated in vivo with immunoglobulins, complement, or both. The direct antiglobulin test is sometimes colloquially called the direct Coombs test because it is based on a test developed by Coombs, Mourant, and Race.
In contrast, the indirect antiglobulin test (IAT), commonly known as the indirect Coombs test, is used to measure antibodies in serum or plasma. A direct antiglobulin test (DAT) is used to detect immunoglobulins and/or complement on the surface of red blood cells (RBCs). DAT is useful in evaluating autoimmune hemolytic anemia, drug-induced hemolysis, hemolytic disease of the newborn, hemolytic transfusion reactions, and passenger lymphocyte syndrome.
DAT has some limitations:
SensitivityFalse Positives and False Negatives.Interpretation of the DAT should also consider patient history, diagnosis, and other laboratory test results.
To learn more about DAT, refer:
https://brainly.com/question/26566066
#SPJ4
When acid is added to pure water, the ph ___________, and when base is added to pure water, the ph ___________.
Answers
The pH of clean water increases when an acid is introduced. The opposite is true if a base is introduced to pure water. A solution's hydronium ion concentration must fall as the concentration of hydroxide ions rises.
Why is the pH of pure water neutral?If there are more hydrogen ions than hydroxide ions in a solution (i.e., pH pOH), the solution is acidic. Since the concentration of hydrogen and hydroxide ions in pure water is constant, even when the pH changes, the water remains neutral (pH = pOH).
Can a base increase water's pH?Bases and Acids
An acid is a chemical that, when introduced to pure water, will cause the pH to fall. In a similar way, a base is something that will raise the pH of water by 4.
To know more about pH of pure water visit:-
https://brainly.com/question/13822050
#SPJ4
what do you think happens to a sound wave when the volume of sound increase
Answers
As the volume of the sound is seen to increase, the sound that we hear is louder as the amplitude of the sound increases.
What is sound?Sound is a kind of wave that moves through a medium. We know that sound has to move via several compressions and rare factions. This implies that sound is as well propagated through air. The movement of the sound waves through air is because the air is set into vibration by the sound. Thus, the air is very imprtant in the movement of the sound waves.
With this much said, we can see that if there is more air, there would more molecules that can be set into vibration and the sound wave would tend to increase and that is the deal that we are trying to communicate here.
Thus, the amplitude of air increases as the volume of the sound gets higher and the sound that we hear is much louder.
Learn more about sound waves:https://brainly.com/question/21995826
#SPJ1
Determine the correct characteristics to recognize a covalent compound.
Answers
Covalent bonds are formed by sharing electrons. Covalent compounds are also known as molecular compounds, and they typically have low melting and boiling points. These are some characteristics that can help identify covalent compounds:Electron Sharing: Covalent compounds are formed when two or more atoms share valence electrons with one another.
Atoms with similar electronegativity will tend to share electrons, which leads to the formation of covalent bonds. Covalent bonds can be polar or nonpolar, depending on the difference in electronegativity between the two atoms involved in the bond.Low Melting and Boiling Points: Covalent compounds generally have lower melting and boiling points than ionic compounds. This is because covalent compounds are held together by weak intermolecular forces rather than strong electrostatic forces. This makes them easier to melt or boil.Molecular Shape: Covalent compounds are typically made up of discrete molecules that are held together by covalent bonds. The shape of these molecules is determined by the arrangement of their atoms and the number of lone pairs of electrons around the central atom.Electrical Conductivity: Covalent compounds do not conduct electricity in the solid or liquid state, but they can conduct electricity when dissolved in water or other polar solvents. This is because the water molecules can break apart the covalent bonds and create ions that are able to carry an electric charge.
For more information on Covalent bonds visit:
brainly.com/question/19382448
#SPJ11
Why do the protons identify the element and not the electrons or the
neutrons?
Answers
Can someone help me Balance chemical equations please

Answers
Hopes this helps <33
In the hypothetical reaction below, substance A is consumed at a rate of 2. 0 mol/L·s. If this reaction is at dynamic equilibrium, at what rate will substance B be consumed? A Double headed arrow. B 0. 0 mol/L·s 1. 0 mol/L·s 2. 0 mol/L·s 4. 0 mol/L·s.
Answers
A chemical reaction is in dynamic equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction.
The correct answer is 2.0 mol/L·s .
The concentrations of the reactants and products in the equilibrium state are known as the equilibrium concentrations. The rate of the forward and backward reaction in equilibrium state is equal; there is no net change in the quantity of reactants or products present in the equilibrium state. However, the reaction continues. In other words, the chemical reaction is still active; however, the quantity of the product and reactant stays the same at equilibrium.
In dynamic equilibrium, the rate of forward reaction is equal to the rate of the backward reaction. The chemical reaction is still active, but the concentration of the products and reactants remain the same. The balanced chemical reaction can be represented as follows: A ⇋ BThe above chemical reaction can be in a state of dynamic equilibrium when the rate of the forward reaction is equal to the rate of the backward reaction.
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
(0)
Calculate the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K.
Give your answer in kJ/mol
_________________________
Answers
The standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Given: ΔT = 15 K and ∆K/K = 1/2
Temperature is directly proportional to equilibrium constant K,
So, ∆T/T = ∆K/K
This can be written as ∆K/K = ΔH°/R × (1/T2 − 1/T1)
On solving this equation, we getΔH° = −2.303 × R × ΔK/K × T2T2 = 310 + 15 = 325 K∆K/K = 1/2∆H° = −2.303 × 8.314 J mol−1 K−1 × 1/2 × 325 K∆H° = −955.7 J mol−1= −0.956 kJ/mol
Therefore, the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Learn more about enthalpy with the given link,
https://brainly.com/question/14047927
#SPJ11
Moraines left by glaciers are different from deposits left by rivers because the rocks left behind are _____.
Answers
Explanation:
unorganized and unsorted i think this is the answer
PLEASEE HELPPP!!!! In a particular reaction between copper metal and silver nitrate, 12. 7 g AgNO3 produced 4. 57 g Ag. What is the percent yield of silver in this reaction?
Answers
To calculate the percent yield of silver in the reaction between copper metal and silver nitrate, we need to compare the actual yield of silver (4.57 g) to the theoretical yield of silver based on the stoichiometry of the reaction.
First, we need to determine the balanced chemical equation for the reaction. It is given as:
Cu + 2 AgNO3 → 2 Ag + Cu(NO3)2
From the balanced equation, we can see that 1 mole of copper reacts with 2 moles of silver nitrate to produce 2 moles of silver.
To find the theoretical yield of silver, we need to calculate the amount of silver that would be produced if all the silver nitrate reacted completely. We can do this by converting the mass of silver nitrate (12.7 g) to moles using its molar mass and then using the stoichiometry of the reaction to find the moles of silver produced.
The molar mass of AgNO3 is:
AgNO3: 107.87 g/mol + 14.01 g/mol + (3 * 16.00 g/mol) = 169.87 g/mol
Moles of AgNO3 = mass / molar mass
Moles of AgNO3 = 12.7 g / 169.87 g/mol ≈ 0.0748 mol
From the stoichiometry, we know that 1 mole of AgNO3 produces 2 moles of Ag. Therefore, the theoretical yield of silver would be:
Theoretical yield of Ag = 0.0748 mol AgNO3 * (2 mol Ag / 1 mol AgNO3) = 0.1496 mol Ag
Now we can calculate the percent yield using the actual yield and theoretical yield:
Percent yield = (Actual yield / Theoretical yield) * 100
Percent yield = (4.57 g / 0.1496 mol) * 100 ≈ 3055%
The percent yield of silver in this reaction is approximately 3055%. It is important to note that a percent yield greater than 100% suggests a potential error in the measurements or experimental procedure.
Learn more about copper metal and silver nitrate here:
https://brainly.com/question/15088874
#SPJ11
Give the coordination number of the metal ion and the number of ions per formula unit in each compound in Problem 23.58.
Answers
A coordination complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bond to a central metal atom (or ion) by coordinate covalent bonds.
What is a metal ion?A metal ion is a type of atom compound that has an electric charge. Such atoms willingly lose electrons in order to build positive ions called cations. Ions are essentially enclosed by delocalized electrons which are responsible for processes like conductivity.
In an ionic crystal, the coordination number of a metal ion (+ve ion) is the number of negative ions surrounding the metal ion, i.e. which are present as its nearest neighbors.
A coordination complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bond to a central metal atom (or ion) by coordinate covalent bonds. Ligands are Lewis bases - they contain at least one pair of electrons to donate to a metal atom/ion.
To learn more about the coordination number of metal ions refer to:
https://brainly.com/question/23129240
#SPJ4
why does the equilibrium strongly favor the reverse reaction hydration of the alkene
Answers
The equilibrium for the hydration of alkenes strongly favors the reverse reaction due to thermodynamic factors.
When an alkene is hydrated, an alcohol is formed by adding water across the double bond. This reaction is exothermic and releases energy. As a result, the entropy of the system decreases since fewer molecules are present in the products than in the reactants.
In contrast, the reverse reaction is endothermic and requires energy to proceed. This results in an increase in the entropy of the system since more molecules are present in the products than in the reactants.
Therefore, the equilibrium for the hydration of alkenes strongly favors the reverse reaction due to the thermodynamic factors of energy and entropy.
You can learn more about reverse reaction at
https://brainly.com/question/14611993
#SPJ11
1. What is the molarity of a Mg(OH)2 solution if 30 mL is required to neutralize 85 mL
of a 2.0 M solution of HNO3?
Answers
Answer:
A solution is a homogeneous mixture of two or more chemical substances. If we have a solution
made from a solid and a liquid, we say that the solid is dissolved in the liquid and we call the
solid the solute and the liquid the solvent. Initially, we will consider only solutions of a solid in
water. If a solution has a small amount of solute in a large amount of solvent, we say that the
solution is dilute (or that we have a dilute solution). If a solution has a large amount of solute
for a certain amount of solvent, we say that the solution is concentrated (or that we have a
concentrated solution). We see that the terms dilute and concentrated are not precise and are
merely used to give a rough indication of the amount of solute for a given amount of solvent.
The amount of solute in a given amount of solvent (or solution) is called the concentration of
the solution. In this course, we will consider two ways of expressing concentration - mass
percent and molarity. We will consider molarity here and mass percent later.
Explanation:
Why is it important for lab safety to know the proper names of equipment in a lab
Answers
Answer:
Knowing your lab equipment and their names will aid in having a successful experiment and may help in correcting errors.
If you do not know your lab equipment, that will only result in having a lack of knowledge of the equipment or not knowing how to correct a mistake in an experiment.
Explanation:
Hope I helped.
The seven diatomic elements (exist as two atoms bonded together in nature) are hydrogen (H2), Nitrogen (N2), Oxygen (O2), Fluorine (F2), Chlorine (C2), Iodine (I2) and Bromine (B2). Based on the position of the seven elements that are diatomic when pure in nature and the trends of the periodic table, which of the following characteristics would not describe them?
a) Smaller Atomic Radius
b) High Metallic Character
c) High Ionization Energy
d) High Electron Affinity
Answers
Answer:
Smaller Atomic Radius, High Ionization Affinity, and High Electron Affinity
[Note: It is Br2, not B2, the bomber]
Explanation:
a) Smaller Atomic Radius YES Each of these elements have a high number of electrons that nearly fill the lower energy orbitals, forcing the protons in the nucleus to cluster togeter more tightly.
b) High Metallic Character NO
c) High Ionization Energy YES - They want to keep their electrons, so the ionization energy is high.
d) High Electron Affinity YES They attract electons from anything that comes nearby.
Given the following reaction: C4H8 < == > C4H6 + H2. If steam is added to the feed and everything else kept the same, the equilibrium conversion to hydrogen ___________.
Group of answer choices
increases
decreases
remains the same
Answers
The equilibrium conversion to hydrogen decreases when steam is added to the feed and everything else kept the same for the following reaction: C₄H₈ < == > C₄H₆ + H₂.
Conversion is defined as the transformation of a compound into another, as a result of a chemical reaction. Equilibrium conversion is the extent of conversion when a chemical reaction proceeds to equilibrium. For the given reaction:C4H8 < == > C₄H₆ +H₂
The conversion of C₄H₈ to C₄H₆ and H₂ is decreased when steam is added to the feed and everything else kept the same. The decrease in conversion is due to the Le Chatelier's principle. It states that when a system in chemical equilibrium is subjected to a change in temperature, pressure, or concentration of reactants or products, the system shifts in equilibrium position to counteract the effect of the change.
As per the principle, the system will counteract the increase in the concentration of water molecules by moving in the direction that reduces the concentration of water molecules, that is, backward.
This causes the reverse reaction to occur more and reduces the conversion of C₄H₈ to C₄H₆ and H₂. Hence, the equilibrium conversion to hydrogen decreases when steam is added to the feed and everything else kept the same.
To know more about equilibrium conversion, refer
https://brainly.com/question/32138537
#SPJ11
Why aren't 3rd 4th and 5th compounds having same stability?

Answers
Answer:
Due to cis trans isomerism
Explanation:
Directions: Identify each chemical equation as a synthesis, decomposition,
single-displacementor, or double displacement reaction.
a. Synthesis b. Decomposition c. Single Displacementor
d. Double Displacement
9.2H2O(→ 2H2(9 +0f9
10.0129 +2KBr(aq) + 2KBr(aq) + Bry()
11. CaO3 + H2O() - Ca(OH)() (
12. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O()
+

Answers
Answer: 9. decomposition 10. single displacement 11. synthesis 12. double displacement
Explanation: just know I know
A sample of propane, a component of lp gas, has a volume of 35. 3 l at 315 k and 922 torr. What is its volume at stp?.
Answers
We assume that the gas is an ideal gas in order to solve this. The ideal gas equation, which is written as PV = nRT, can then be used. (922/760) x 35.3/315 = 1 x V2 /273. V2 = 37.1L. As a result, choice D) is correct.
STP, 1 mole of gas occupies a volume of 22.4 L (molar volume). At standard temperature and pressure (STP), molar volume (Vm) is the volume occupied by one mole of a chemical element or compound. It can be calculated by dividing the molar mass (M) by the mass density (ρ). Gas volume is defined as the space occupied by gas particles at standard temperature and pressure conditions. Represented by a "V". The SI unit of volume is the liter, denoted L. One mole of gas has a volume of 24 m3 or 24000 cm3 at room temperature. This value is called the molar volume of the gas.
Learn more about pressure here-
https://brainly.com/question/12971272
#SPJ4
If a car rolled down a 20 meter ramp,continued rolling at the bottom and eventually came to a stop 15 meters later, what is the car’s displacement ? What is the car distance traveled?
Answers
Answer:
See attached.
Explanation:
The distance and the displacement are the measurements of the object that traveled. The distance traveled by the rolling car is 5 m and the displacement is 5 m downwards.
What are distance and displacement?Distance has been the area covered by the object from the initial to the final point it has no directions. It estimates the points from where the object moved to reach the destination. The distance traveled is calculated as:
Distance = 20 m - 15 m
D = 5 m
The displacement is the overall movement and change in the position of the object irrespective of the area covered. It is a vector quantity and is measured as:
D = Δx
D = X₂ - X₁
D = 20 m - 15 m
D = 5 m downwards
Therefore, the displacement of the car is 5 meters downwards and the distance is 5 meters.
Learn more about distance and displacement, here:
https://brainly.com/question/3243551
#SPJ2
what are local winds. be more detailed. and in your own words
Answers
Answer:
High and low pressure regions form when there is a large temperature difference between the surface of the sea (or a large lake) and the land next to it. Local winds are created as a result of this. Warmer air from the ocean rises and then sinks on land, causing the temperature to rise over the land.
i only need five and six please and thank you
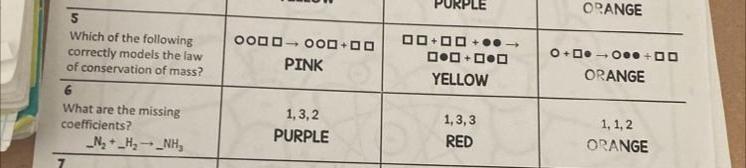
Answers
Answer:
Yellow, Purple
Explanation:
5. The law of conservation of mass essentially states that the total 'mass' that you started with equals to the total 'mass' at the end. Take the burning of a tree, for example. Although the tree loses mass, the mass of carbon dioxide and other molecules increases proportionally to the rate of tree mass loss. Abiding by this law in a chemical reaction, we should expect the amount of shapes on each side to be equal, although they may not necessarily be in the same form. This is only shown in the 'Yellow' square.
6. We want the same amount of stuff on the left as we do on the right. There is 2 Nitrogen and 2 Hydrogen on the left. There is 1 Nitrogen and 3 Hydrogen on the right. To balance these amounts out, we'd need to multiply a few things:
1 Nitrogen by 2 on the right to make things even, meaning that now there is 2 Nitrogen and 6 Hydrogen on the right. To balance this out on the left, we need to multiply the 2 Hydrogen by 3 to yield 6 Hydrogen.
As such, the new equation is N2+ 3H2 --> 2NH3.
Do we have the same amount of stuff on the left as we do on the right? Yes! So the equation is balanced.
Is the copper in a copper wire a homogeneous or heterogeneous mixture
Answers
If you have the following data about a jar of jellybeans, about how many jellybeans are estimated to be in the jar?
Mass of Jellybeans + Jar = 1642 grams
Mass of 20 Jellybeans = 7.94 grams
Mass of Jar ONLY = 495 grams Approximately, how many jellybeans are in the jar?
Answers
First, we need to calculate the mass of jellybeans alone.
Mass of Jellybeans + Jar = 1642 grams
Mass of Jar ONLY = 495 grams
Mass of Jellybeans = Mass of Jellybeans + Jar - Mass of Jar ONLY
Mass of Jellybeans = 1642 g - 495 g
Mass of Jellybeans = 1147 g
Next, we need to find the mass of one jellybean.
Mass of 20 Jellybeans = 7.94 grams
Mass of 1 Jellybean = Mass of 20 Jellybeans ÷ 20
Mass of 1 Jellybean = 0.397 g
Finally, we can estimate the number of jellybeans in the jar by dividing the total mass of jellybeans by the mass of one jellybean.
Number of Jellybeans = Mass of Jellybeans ÷ Mass of 1 Jellybean
Number of Jellybeans = 1147 g ÷ 0.397 g
Number of Jellybeans ≈ 2891
Therefore, there are approximately 2891 jellybeans in the jar.
Answer:
mass of jellybeans + jar = 1642 g
mass of jar = 495 g
mass of jellybeans alone = 1642 - 495 = 1147g
mass of 20 jellybean= 7.94 g
mass of 1 jellybean= 7.94 ÷ 20 = 0.397 g
total number of jelly beans = 1147 ÷ 0.397 = 2889
There are approximately 2889 jellybeans in jar