some students using Apple to represent the Earth what is the best reason to use an Apple for this comparison?
Answers
Answer:
The skin of the apple is very thin, and the crust of the Earth is very thin.
Related Questions
ethanol, c2h5oh , will combust in air according to the equation above. (a) is o2(g) oxidized in the reaction, or is it reduced? justify your answer in terms of oxidation numbers.
Answers
In the ethanol, C₂H₅OH , will combust in the air is the O₂(g) is reduced.
The chemical equation is as :
C₂H₅OH (l) + 3O₂ (g) ----> 2CO₂ (g) + 3H₂O (g) ΔH° = –1270 kJ/mol
The oxidation is the increase in the oxidation number. In the chemical reaction that is undergoing the oxidation and if there will be the positive increase in the oxidation number from the left to the right in the reaction.
The oxidation numbers of the elements in the chemical reaction, the oxygens in the O₂ (g) is zero. The oxygens in the both CO₂ (g) and the H₂O (g) are the -2. Therefore the oxidation number of the O₂ decrease and is called as reduction or it is reduced.
To learn more about reduction here
https://brainly.com/question/4222605
#SPJ4
Shelton measures a temperature of a solution to be 20O degrees C and stirs 5 grams of a substance into the solution. this substance disappears and 10 minutes later the substance of the solution is 80O degrees C.
based on his observation, can Shelton conclude a chemical reaction has occurred?
A) yes because the substance dissolved in the solution
B) no because the volume of the solution did not change
C) no because there are no color changing solution
D) yes because heat was produced

Answers
Answer:
did u ever get an answer??
Explanation:
Answer:
The answer is C. Yes, because heat was produced
Students were responsible for conducting 4 trials of titration of 15 mL samples of phosphoric acid (H3PO4) with volumes of a 2.2 molar strontium hydroxide (Sr(OH)2) solution. Determine the concentration of the phosphoric acid solution using the results that were obtained.
2H3PO4(aq) + 3Sr(OH)2(aq) Sr3(PO4)2(s) + 6H2O(l)
Trial 1
Trial 2
Trial 3
Trial 4
Initial Buret Reading (mL)
0.1
12.2
24.4
36.1
Final Buret Reading (mL)
12.2
24.4
36.1
48.3
Volume Base Added (mL)
Answers
The concentration of the phosphoric acid solution using the obtained results would be 1.2 M.
Stoichiometric problemFirst, we need to determine the volume of base used for each trial.
Volume of base used = final - initial burette reading
trial 1 = 12.2 - 0.1 = 12.1 mLtrial 2 = 24.2 - 12.2 = 12.0 mLtrial 3 = 36.1 - 24.4 = 11.7 mLtrial 4 = 48.3 - 36.1 = 12.2 mlTitre value = 12.1+12.0+12.2/3 = 12.1 mL
From the balanced equation of the reaction: \(2H_3PO_4(aq) + 3Sr(OH)_2(aq) -- > Sr_3(PO_4)_2(s) + 6H_2O(l)\)
The mole ratio of the phosphoric acid to the strontium hydroxide is 2:3.
Mole of 2.2 M, 12.1 mL strontium hydroxide = 2.2 x 12.1/1000
= 0.027 mol
The equivalent mole of phosphoric acid = 2/3 x 0.027
= 0.018 mol
Concentration of 15 mL, 0.018 mol phosphoric acid = 0.018/0.015
= 1.2 M
In other words, the molarity of the phosphoric acid is 1.2 M.
More on stoichiometric problems can be found here: https://brainly.com/question/15047541
#SPJ1
3H2 + blank, reaction arrow, 2NH3
How to complete this equation so that it is a Synthesis reaction
Answers
The reaction is completed by the equation below: \(3H_2 + N_2 --- > 2NH_3\)
What are synthesis reactions?They are chemical reactions in which two or more molecules react to produce a single product.
In this case, the synthesis reaction is that of ammonia. Molecules of hydrogen gas and nitrogen gas combine to produce ammonia.
The equation of the reaction is as follows: \(3H_2 + N_2 --- > 2NH_3\)
More on synthesis reactions can be found here: https://brainly.com/question/24936069
#SPJ1
A reactant decomposes with a half-life of 115 s when its initial concentration is 0.288M. When the initial concentration is 0.761 M, this same reactant decomposes with the same half-life of 115 s. What is the order of the reaction? 5 1 0 What is the value and unit of the rate constant for this reaction? k= Units:
Answers
The order of the reaction can be determined by comparing the concentration change with respect to time for different initial concentrations of the reactant. In this case, the half-life remains the same (115 s) for both initial concentrations, indicating that the reaction is first order. The unit of the rate constant depends on the overall reaction order. Since the reaction is first order, the unit of the rate constant would be reciprocal seconds, or s^(-1).
The rate constant (k) for a first-order reaction can be determined using the equation:
k = ln(2) / t1/2
where t1/2 is the half-life of the reaction. Plugging in the given value of t1/2 = 115 s, we can calculate the rate constant:
k = ln(2) / 115 s ≈ 0.00602 s^(-1).
The given problem involves determining the order of a reaction and calculating the rate constant. By comparing the concentration change with respect to time for different initial concentrations, it is observed that the reactant exhibits a consistent half-life of 115 seconds. This indicates that the reaction is first order.
For a first-order reaction, the rate constant (k) can be calculated using the equation k = ln(2) / t1/2, where t1/2 represents the half-life. Plugging in the provided value of t1/2 = 115 seconds, the rate constant is determined to be approximately 0.00602 s^(-1).
The unit of the rate constant depends on the reaction order. Since the reaction is first order, the unit of the rate constant is reciprocal seconds, or s^(-1). This unit reflects the rate at which the reactant decomposes over time.
To know more about rate constant, visit;
https://brainly.com/question/20305922
#SPJ11
Perform Calculatlons Using Ksp Question For the following equilibrium; HgBr2(s) ~= Hg?+(aq) + 2Br"(aq) If Ksp 6.2 x 10-20, what is the molar solubility of mercury (II) bromide (HgBrz)? Report your answer in scientific notation with the correct number of significant figures Provide your answer below:'
Answers
The molar solubility of mercury (II) bromide (HgBr2) is 7.00 × 10^-7 M. This is the long answer to the problem.
To perform calculations using Ksp for the given equilibrium HgBr2(s) ↔ Hg2+(aq) + 2Br-(aq) and find the molar solubility of mercury (II) bromide (HgBr2) we have to use the solubility product expression.Ksp for HgBr2 is 6.2 × 10^-20Ksp = [Hg2+][Br-]^2 Since the initial concentration of HgBr2 is given as s, and after dissociation, the concentration of Hg2+ becomes s, while the concentration of Br- becomes 2s.[Hg2+] = s M and [Br-] = 2s MThus,Ksp = [Hg2+][Br-]^2= s(2s)^2= 4s^3= 6.2 × 10^-20Molar solubility of HgBr2 is given as s, therefore;s = (6.2 × 10^-20/4)1/3s = 7.00 × 10^-7 M
To know more about molar solubility visit:-
https://brainly.com/question/31043999
#SPJ11
What would the mass be, in grams, of 0.89 moles of Cl2?
Answers
Answer:
Mass = 63.19 g
Explanation:
Given data:
Number of moles of Cl₂ = 0.89 mol
Mass in gram = ?
Solution:
Formula:
Mass = number of moles × molar mass
Molar mass of Cl₂ = 71 g/mol
by putting values,
Mass = 0.89 mol × 71 g/mol
Mass = 63.19 g
A chemist, Dr. V.A. Pore, wishes to detect an impurity in a certain compound that she is making. There is a test that has sensitivity of 0.9 and specificity of 0.95. That is, the test is positive for an impurity when an impurity is present 90% of the time, and the test is negative for an impurity when no impurity is present 95% of the time. About 15\% of Dr. Pore's compounds contain an impurity. 9. A compound is selected at random from Dr. Pore's output. The test indicates that an impurity is present. What is the conditional probability that the selected compound actually has an impurity? 1 10. Another compound is selected at random from Dr. Pore's output. The test indicates that an impurity is not present. What is the conditional probability that the selected compound is actually free of an impurity? 11. Two processes of a company produce rolls of materials. The rolls of Process I are 3% defective, and the rolls of Process II are 1% defective. Process I produces 60% of the company's output, Process II 40%. A roll is selected at random from the total output. Given that this roll is defective, what is the conditional probability that it is from Process I?
Answers
The correct options are:1.
Conditional probability that the selected compound actually has an impurity is 0.74.2.
Conditional probability that the selected compound is actually free of an impurity is 0.0185.3.
Conditional probability that the selected roll is from Process I given that it is defective is 0.64.
Here, we need to find out the probability that a selected compound has an impurity given that the test indicates an impurity is present.
P(A) = probability that a compound has impurity = 0.15
P(B) = probability that the test indicates an impurity is present
= 0.15 x 0.9 + 0.85 x 0.05
= 0.14 + 0.0425
= 0.1825P
(B|A) = probability that the test indicates an impurity is present given that the compound has impurity = 0.9
Therefore, by Bayes' Theorem,
P(A|B) = P(B|A) * P(A) / P(B)
= 0.9 * 0.15 / 0.1825
= 0.7370
≈ 0.74
Conditional probability that the selected compound actually has an impurity is 0.74.10.
Here, we need to find out the probability that a selected compound is actually free of an impurity given that the test indicates an impurity is not present.
P(A) = probability that a compound has impurity = 0.15
P(B) = probability that the test indicates an impurity is not present = 0.85 x 0.95 + 0.15 x 0.1 = 0.8075
P(B|A) = probability that the test indicates an impurity is not present given that the compound has impurity
= 0.1
Therefore, by Bayes' Theorem,
P(A|B) = P(B|A) * P(A) / P(B)
= 0.1 * 0.15 / 0.8075
= 0.0185
Conditional probability that the selected compound is actually free of an impurity is 0.0185.11.
Here, we need to find out the probability that the selected roll is from Process I given that it is defective.
Let A denote the event that a roll is from Process I and B denote the event that a roll is defective.
Then, we need to find out P(A|B).
P(A) = probability that a roll is from Process I = 0.6
P(B|A) = probability that a roll is defective given that it is from Process I = 0.03
P(B|A') = probability that a roll is defective given that it is from Process II = 0.01
P(A'|B) = probability that a roll is from Process II given that it is defective
Therefore, by Bayes' Theorem,
P(A|B) = P(B|A) * P(A) / [P(B|A) * P(A) + P(B|A') * P(A')]
= 0.03 * 0.6 / (0.03 * 0.6 + 0.01 * 0.4)
= 0.6429
≈ 0.64
Conditional probability that the selected roll is from Process I given that it is defective is 0.64.
Hence, the correct options are:1.
Conditional probability that the selected compound actually has an impurity is 0.74.2.
Conditional probability that the selected compound is actually free of an impurity is 0.0185.3.
Conditional probability that the selected roll is from Process I given that it is defective is 0.64.
Learn more about impurity from this link:
https://brainly.com/question/14932983
#SPJ11
How many isotopes does Potassium have?
Here is my work:

Answers
Potassium have three isotopes.
How many different isotopes are there overall?254 stable isotopes are known. Certain substances can only exist in unstable states (for example, uranium).
The stable potassium isotope has a nuclear spin of 3/2, a relative atomic mass of 38.963707, and a natural abundance of 93.3 atoms.
We must compute for the various atomic weights of the isotopes since potassium has three distinct isotopes: Potassium-39 has an atomic mass of 39 and 19 protons. Therefore, the number of neutrons is 39 – 19, or 20.
If potassium has an atomic mass of 39.0983, the average is obviously highly biassed in favour of potassium-39, making potassium-39 the isotope with the greatest abundance.
learn more about isotope refer
https://brainly.com/question/14220416
#SPJ13
What are all the possible intermolecular forces between acetone and water?.
Answers
Answer:
Well, the acetone has a dipole, so dipole-dipole forces will be present. Water has a dipole and can also hydrogen bond, as can isobutyl alcohol.
Explanation:
All the possible intermolecular forces between acetone and water will be dipole - dipole interaction force and hydrogen bond.
What is dipole - dipole interaction force?The attractive interactions between both the positive ends of one polar molecule as well as the negative ends of another polar molecule were known as dipole-dipole forces.
What is hydrogen bond?
A hydrogen bond seems to be an electrical attraction between such a hydrogen atom covalently bonded to a much more electronegative "donor" atom and group and maybe another electronegative with a single pair of electrons.
Acetone possesses a dipole, there must be dipole-dipole forces. Isobutyl alcohol, like water, does have a dipole and therefore can hydrogen bond.
To know more about hydrogen bond and dipole dipole interaction.
https://brainly.com/question/7545068
#SPJ2
An isotope has a half-life of 12.5 years. How long must you wait for the radioactivity rate to decrease to 14.35% of its original value?
Answers
An isotope has a half-life of 12.5 years, you must wait for 36.5 years for the radioactivity rate to decrease to 14.35% of its original value
The amount of radioactive material decreases by half during each half-life. Therefore, if the radioactivity rate decreases to 14.35% of its original value, it means that the material has gone through a certain number of half-lives.
We can use the following formula to determine the number of half-lives:
n = log(1/2) / log(1 + r)
where n is the number of half-lives, r is the fraction of the original material remaining (in this case, 0.1435), and log represents the logarithm with base 10.
Plugging in the values, we get:
n = log(1/2) / log(1 + 0.1435) ≈ 2.92
This means that the material has gone through approximately 2.92 half-lives. Since the half-life is 12.5 years, the total time elapsed would be:
t = n x half-life = 2.92 x 12.5 years ≈ 36.5 years
Therefore, you would need to wait for approximately 36.5 years for the radioactivity rate to decrease to 14.35% of its original value.
To know more about radioactivity rate, refer here:
https://brainly.com/question/29812745#
#SPJ11
propose one chem 212 reaction that may be made more efficient using mw heating
Answers
By utilizing microwave heating in the Fischer esterification reaction, researchers can achieve shorter reaction times, higher yields, and improved reaction efficiency compared to traditional heating methods.
What is heating methods?“Heating” is a traditional technique known to humanity. It has been everchanging the way we live. There are an innumerable ways in which we have been generating heat and using the heat to do work. One of the key applications of “heat” has been the Industrial Process Applications.
One reaction that may be made more efficient using microwave (MW) heating is the synthesis of esters through the Fischer esterification reaction.
In the Fischer esterification, an alcohol reacts with a carboxylic acid in the presence of an acid catalyst to form an ester. The traditional heating method for this reaction involves refluxing the reaction mixture for several hours to achieve reasonable yields.
Using microwave heating in the Fischer esterification reaction can significantly reduce the reaction time and increase the reaction efficiency. MW heating provides rapid and efficient heating by directly transferring energy to the reactants, resulting in faster reaction rates and higher yields.
The MW heating method allows for more precise temperature control and selective heating of the reaction mixture. It can also promote better mixing and faster mass transfer, which enhances the reaction kinetics and reduces the formation of undesired by-products.
Overall, by utilizing microwave heating in the Fischer esterification reaction, researchers can achieve shorter reaction times, higher yields, and improved reaction efficiency compared to traditional heating methods.
Learn More About heating methods
https://brainly.com/question/32051311
#SPJ4
What formation results when a river deposits sediment and it slows down as it enters a large body
of water?
Group of answer choices
a. dune
b. delta
c. bay
d. alluvial fan
Answers
Answer:
b
Explanation:
name a metal that would be magnetic
Answers
In the equation shown, what represents the concentrations of the products?
K = (W)(X)/(Y)(Z)

Answers
In the equation K = (W)(X)/(Y)(Z), the variables W, X, Y, and Z represent the molar concentrations of the reactants and products involved in the chemical reaction.
Specifically, W represents the molar concentration of the first reactant, X represents the molar concentration of the second reactant, Y represents the molar concentration of the first product, and Z represents the molar concentration of the second product.
The equation molarity = (mass solute / MW) / L solution is used to calculate the molar concentration of a solute in a solution. In this equation, "mass solute" refers to the mass of the solute dissolved in the solution, "MW" refers to the molecular weight of the solute, and "L solution" refers to the volume of the solution.
Note that while both equations involve concentrations, they are not directly related. The first equation is a thermodynamic expression of the equilibrium constant of a chemical reaction, while the second equation is used to calculate the concentration of a solute in a solution.
Learn more about Concentrations at
brainly.com/question/10725862
#SPJ1
If pesticides must be stored on the premises, which location is the best choice?Gnaw marks on wood, metal, or concreteIn a closet with cleaning chemicalsSome chemicals are not approved for use in foodservice operations.
Answers
The best choice for storing pesticides on the premises would be a location that is secure and separate from other chemicals or materials.
Pesticides are potentially hazardous chemicals that can cause harm to humans and animals if they are not handled properly. Therefore, it is important to store them in a secure location that is inaccessible to unauthorized personnel.
Gnaw marks on wood, metal, or concrete could indicate the presence of pests, which could lead to contamination of the pesticides. A closet with cleaning chemicals may not be the best choice, as mixing cleaning chemicals with pesticides could create a dangerous reaction.
Additionally, some chemicals are not approved for use in foodservice operations, so it would not be appropriate to store them in areas where food is prepared or stored. The best choice for storing pesticides would be a designated area that is secure, well-ventilated, and away from other chemicals or materials that could potentially cause a reaction or contamination.
To know more about pesticides refer here:
https://brainly.com/question/31600813#
#SPJ11
Mutations are changes in the genes of living things. Mutations are common in all life forms and usually harmless. In fact, sometimes they help organisms survive. However, people often think that the word mutation means something dangerous. Which best describes the question "Why do most people think mutations are dangerous?" a scientific question, because it asks about mutations that happen in nature a scientific question, because it asks about most people instead of just a few not a scientific question, because it asks about what people think instead of how nature works not a scientific question, because it asks about how mutations can be helpful to life in nature (ANSWER IT FIRST AND GET BRAINLIST)
Answers
Answer: A
Explanation:
Answer:
C
Explanation:
What volume (in mL) of 0.750 M sodium hydroxide is needed to neutralize (react with) 275 ml of 0.500 M sulfuric acid (H2SO4)? Write your answer with the proper number of sig figs. Balance the equation first.Your answer will be in mL
NaOH + H2SO4 -> H2O + Na2SO4
I need workkkk
Answers
_ we have C[NaOH]= 0.750M, V[H2SO4]=250ml, C[H2SO4]=0.500M
_ n[H2SO4]= c.v = 0.500x250=125mol
So n[NaOH]= 2n[H2SO4]
= 2x125mol=250mol
So V[NaOH]= n/c = 250ml/0.750M= 333.33ml
9 Dr. Garcia is performing an experiment to see how cell division in flies is affected by the addition of a certain protein, called protein W, into a cell's cytoplasm. She has found that adding protein W to a cell drastically reduces the chances of the cell becoming cancerous. For protein W to be developed into a cancer treatment, what comparison should she make?
Answers
Answer:
The fly cells must be compared with human cells
Explanation:
Dr. Gracia's aim is the development of a potential cancer treatment. Remember that the treatment is meant for human beings.
Usually, the pre-clinical trials of the treatment is carried out on other organisms and the success rate is evaluated before trial on humans.
If protein W has been effective on flies, its effect on human cells should also be diligently compared before conclusions are drawn regarding the therapeutic effects of protein W.
what product of an acid base reaction is an ionic compound
A. water
B. a metal
C. a gas
D. a salt
Answers
The product of an acid-base reaction that is most likely to be an ionic compound is a salt. Option D).
In an acid-base reaction, the product that is most likely to be an ionic compound is a salt. A salt is formed when an acid reacts with a base, resulting in the transfer of ions between the two reactants. Acids typically release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-). When these ions combine, they form water (H2O), which is a neutral molecule.
However, in addition to water, the reaction between an acid and a base also produces a salt. A salt is an ionic compound composed of positive and negative ions. The positive ion usually comes from the base, while the negative ion comes from the acid. The combination of these ions results in the formation of an ionic compound, which is commonly referred to as a salt.
Therefore, the product of an acid-base reaction that is most likely to be an ionic compound is a salt. Hence option D) is correct.
For more question on salt
https://brainly.com/question/20835655
#SPJ8
What happens in the nucleus when a lithium atom becomes an ion
Answers
Answer:
An atom that gains a negative electron, it becomes a negative ion. ... A lithium atom has 3 protons and 3 electrons. It can lose one of its electrons, making it an ion. It now has more positive protons than electrons so it has an overall positive charge.
Answer:
This means a Lithium atom formed into a Lithium ion by losing one of its electrons. It has three electrons and three protons to begin with. So when it loses or gains one the amount isnt equal, that is why it becomes an ion. If it gained electrons it would have a negative charge on the nulcleus, though if it lost electrons it would have a positivec charge. Hope this helped!!
A child sits on a partially filled balloon and decreases the volume by half. What happens to the pressure inside of the balloon?
It increases
It decreases
It stays the same
Answers
**Check for proof photos at the bottom.**
**Answers are in bold.**
__________________________________________________________
A child sits on a partially filled balloon and decreases the volume by half. What happens to the pressure inside of the balloon?
A. It increases
For the same balloon, how does the density inside the balloon change?
A. increases
A scientist takes a small, partially inflated balloon out of liquid nitrogen (at a very low temperature). As the balloon rests on the table, it begins to grow in size. Which statement(s) best explains why the balloon grows?
B. The air warms and increases particle movement. Increased particle movement leads to increased volume.
C. Increased temperature means increased volume as long as the container is flexible.
__________________________________________________________
Answer the following questions with or without using a calculator.
A balloon contains 1.00 mol helium gas at STP. What will the volume of the balloon be if the pressure increases to 2.00 atm?
✔ 11.2 L
A syringe contains 0.10 mol neon gas at STP. What volume does the syringe indicate?
✔ 2.24 L
A chemist adds 0.10 mol argon gas to the syringe. The pressure and temperature remain constant. What will be the volume on the syringe after the argon is added?
✔ 4.48 L
__________________________________________________________
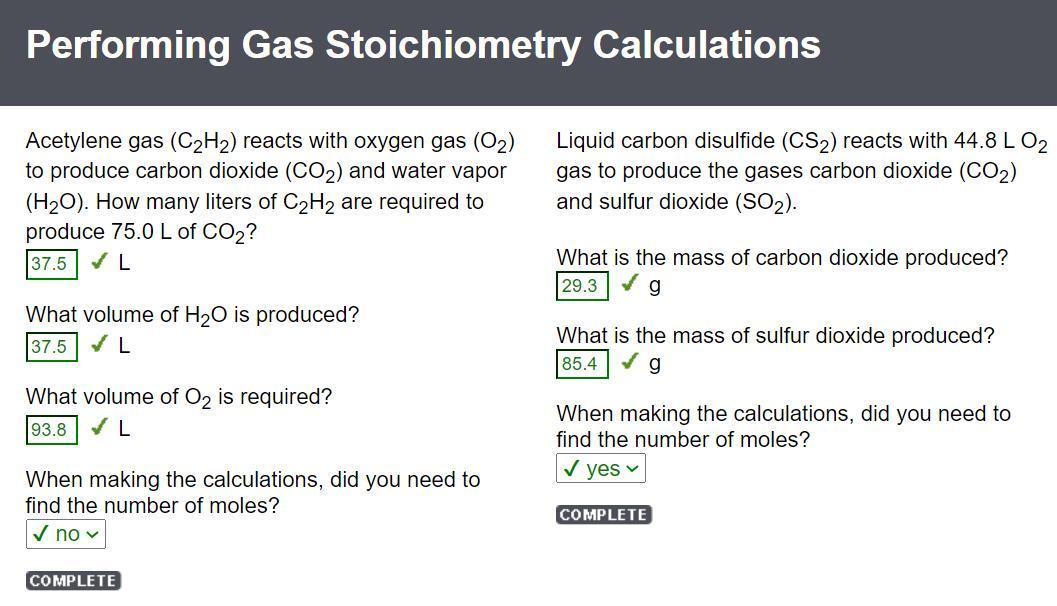
Acetylene gas (C2H2) reacts with oxygen gas (O2) to produce carbon dioxide (CO2) and water vapor (H2O). How many liters of C2H2 are required to produce 75.0 L of CO2?
37.5 L
What volume of H2O is produced?
37.5 L
What volume of O2 is required?
93.8 L
When making the calculations, did you need to find the number of moles?
✔ no
Liquid carbon disulfide (CS2) reacts with 44.8 L O2 gas to produce the gases carbon dioxide (CO2) and sulfur dioxide (SO2).
What is the mass of carbon dioxide produced?
29.3 g
What is the mass of sulfur dioxide produced?
85.4 g
When making the calculations, did you need to find the number of moles?
✔ yes
__________________________________________________________
Explanation:The child is putting pressure on the balloon, and squishing particles together when he/she sits on it, thus increasing pressure. Since particles are being squished closer together, density also increases.
Inflated objects will grow in size when the air inside is hotter. This is because particles move more rapidly. An example would be bike tires being more inflated under hot conditions.
One mole of any gas at standard temperature and pressure (STP) will occupy 22.4 L. If pressure increases, the volume decreases, and vice versa. If moles decreases, so will the volume, and vice versa.
Here are photos of Edge just incase.


The pressure inside of the balloon will Increase. Hence The correct option is (A).
What is Boyle's Law ?
According to this law, the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form,
pv = k
Where K = constant.
Initial pressure = P₁Initial volume = V₁Final pressure =P₂Final volume = V₂If we consider that temperature of the gas which filled inside the balloon is constant.
We know that , at constant temperature
P₁V₁ = P₂V₂
Given that
V₂ = V₁ / 2
P₂ = P₁V₁/V₂
P₂ = P₁V₁/V₁/2
P₂ = 2P₁
Therefore, the pressure inside the balloon will become double.Hence The correct option is (A).
Learn more about Gas Law here ;
https://brainly.com/question/12669509
#SPJ2
In this activity you'll use the Gas Properties PhET Simulation to explore and explain the relationships between energy, pressure, volume, temperature, particle mass, number, and speed. This activity has 5 modules: Explore the Simulation Kinetic Energy and Speed Kinetic Molecular Theory of Gases Relationships between Gas Variables Pressure and Mixtures of Gases You will get the most out of the activity if you do the exploration first! The rest of the sections can be worked in any order; you could work on any sections where you want to deepen your conceptual understanding. Part I: Explore the Simulation Take about five minutes to explore the sim. Note at least two relationships that you observe and find interesting.
Answers
In exploring the Gas Properties PhET Simulation, one interesting relationship that I observed is the relationship between pressure and volume. When the volume of the container holding the gas is decreased, the pressure of the gas increases. Similarly, when the volume is increased, the pressure decreases.
This relationship can be explained through Boyle's Law, which states that at a constant temperature, the pressure of a gas is inversely proportional to its volume.
Another interesting relationship that I observed is the relationship between particle mass and speed. As the mass of the particles increases, their speed decreases. This is because the particles have more mass to move, so they cannot move as quickly as lighter particles. This relationship can be explained through the Kinetic Molecular Theory of Gases, which states that the average kinetic energy of gas particles is directly proportional to their temperature, but inversely proportional to their mass. Overall, exploring the simulation helped me deepen my conceptual understanding of these relationships between gas variables.
In this activity, you will explore the Gas Properties PhET Simulation to understand the relationships between various gas properties. Let's look at two interesting relationships in the context of the Kinetic Molecular Theory of Gases and Speed Kinetic:
1. Relationship between temperature and kinetic energy:
As temperature increases, the average kinetic energy of gas particles also increases. This is because temperature is a measure of the average kinetic energy of the particles in a system. The higher the temperature, the faster the particles move, resulting in greater speed kinetic.
2. Relationship between pressure and volume:
According to Boyle's Law, the pressure of a gas is inversely proportional to its volume when the temperature and the number of particles are constant. This means that when the volume of a gas decreases, the pressure increases, and vice versa. This can be explained by the fact that when the volume is reduced, the gas particles are closer together, resulting in more frequent collisions with the container walls, thus increasing the pressure.
Feel free to explore the simulation and discover more relationships among the gas properties, and use this information to deepen your understanding of the topic.
For more information on Boyle's Law visit:
brainly.com/question/30367067
#SPJ11
Which of the following statements below is TRUE?
Question 18 options:
Never reach across an open flame.
Never heat closed or stoppered glassware.
Never put a glass thermometer in a flame.
All of the above.
Answers
What coefficient would you place in front of O2 in order to balance this chemical equation:
2C2H6 + _?_O2 → 4CO2 + 6H2O
C)8 A) 6 R) 14 B) 7
Answers
Answer:
7 O2
Explanation:
My method involves counting all the elements in the equation. There must be an equal amount of each on both sides. Because it's asking for O2 only, look for compounds that deal with O2
So...CO2 and H2O only on the product side for this chemical equation.
Count the total O there is
CO2 has 2 O in one molecule, so 4 CO2 molecule is 8 Oxygen
H2O has 1 O in one molecule, so 6 H2O molecule is 6 Oxygen.
Your total O on the product side is 14
On the reactant side, the O2 is paired because O2 likes to be stable, so one O2 molecule has 2 O. This means that 2x = 14, which is 7.
Your answer is 7
Answer:
7 O2
Explanation:
You are given the balanced equation 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(ℓ) You are given rules for assigning oxidation numbers in Table 9.3 on page 604 of the student textbook. Compare the oxidation numbers of the atoms of each element on both sides of the equation. ... Therefore, the reaction is a redox reaction.
Scientists digging in a cave found an unknown substance. The scientists
found that the substance’s molecules were moving around each other. The scientists
increased the speed of the substance’s molecules and caused a phase change. How
did the scientists do this, and how did this affect the substance?
Answers
Answer:
By adding heat energy
It changed to gas
Explanation:
A substance whose molecules are moving around each other is a liquid. They are held in shape by very weak intermolecular forces.
For solids, there is no movement because the molecules are held up in a fixed lattice.
Gases molecules moves randomly and independently of one another.
When a liquid is heated and it gains heat energy, it changes phase. A phase change to gas can be predicted. This is because there is an increase in the speed of the substances.2) Usually, when the particle size is decreased, what will happen to the rate of dissoving?
A) It will stop.
B) It will increase.
C) It will decrease.
D) It will remain constant.
Answers
The rate of dissolving is usually affected due to the change of size, temperature of the stirring of the solution.
As the size is decreased it also means that the particles are smaller therefore it will be easier to dissolve. So as a result the rate of dissolving increases
Please please help me with this chemistry question over limiting reactants it’s due by midnight! I’ll mark you BRAINIEST!!!

Answers
Answer: 1.5 moles
Explanation: one mole Zn uses 2 moles HCl.
1.5 moles Zn uses 3.0 mol HCl. Then Zn is a limiting reactant
And produces equal amount of H2.
how much energy is used to raise the temperature of 3kg of aluminium from 18°C to 23°C? Use the table below and this equation: Q=mc T
A. 13,455 J
B. 2691 J
C. 13.455
D. 4.485 J

Answers
How much energy is required to raise the temperature of 3 kg of aluminum from 18°C to 23°C? Use the table below and this equation: Q=mcΔT

Let G and H be groups. Prove if φ(g) = eH for all g ∈ G, the map φ: G to H is a group homomorphism
Answers
φ(g1 * g2) = eH = φ(g1) * φ(g2).
This completes the proof that φ: G → H is a group homomorphism.
By showing that the map φ preserves the group operation, we have demonstrated that it is a group homomorphism.
To prove that φ: G → H is a group homomorphism, we need to show that it preserves the group operation. In other words, for any two elements g1 and g2 in G, φ(g1 * g2) = φ(g1) * φ(g2), where * denotes the group operation in G, and * denotes the group operation in H.
Given that φ(g) = eH for all g ∈ G, where eH is the identity element in H, we can start the proof as follows:
Let g1, g2 ∈ G. We want to show that φ(g1 * g2) = φ(g1) * φ(g2).
Since φ(g) = eH for all g ∈ G, we have φ(g1) = eH and φ(g2) = eH.
Now, consider the product g1 * g2 in G. Applying φ to both sides, we have:
φ(g1 * g2) = φ(g1) * φ(g2).
Substituting the values of φ(g1) and φ(g2), we get:
φ(g1 * g2) = eH * eH.
Since eH is the identity element in H, the product eH * eH is simply eH.
To know more about homomorphism
https://brainly.com/question/6111672
#SPJ11