Problems with understanding what happens when things burn
Answers
Problems with understanding what happens when things burn can be attributed to various factors, such as lack of knowledge about the combustion process, the role of oxygen, and the production of heat and light energy.
When things burn, a chemical reaction called combustion takes place. During this process, a fuel reacts with oxygen, resulting in the release of energy in the form of heat and light. The products of combustion are usually water, carbon dioxide, and sometimes other gases or particles, depending on the fuel and the burning conditions.
One issue in understanding this process is grasping the importance of oxygen. Oxygen is required for combustion to occur, and the presence of more or less oxygen affects the burning process. For example, in a well-ventilated area, the combustion is more efficient, whereas limited oxygen can result in incomplete combustion and the production of harmful byproducts like carbon monoxide.
Another problem in understanding combustion is the role of heat. Heat is both a product of and a catalyst for combustion. As a fuel gets heated, it may reach its ignition temperature, at which point it spontaneously ignites. Heat also contributes to the spread of fire, as it can cause nearby objects to reach their ignition temperature.
The production of light during combustion is another aspect that can cause confusion. The light emitted during burning is a result of excited atoms and molecules in the flame that release energy in the form of light when they return to their original state. This is what makes flames visible and gives them their characteristic colors.
In summary, problems with understanding what happens when things burn stem from a lack of knowledge about the combustion process, the role of oxygen, and the production of heat and light energy. Gaining a deeper understanding of these factors can help individuals better comprehend the complex nature of combustion and fire safety.
To know more about things burn refer here
brainly.com/question/29886555#
#SPJ11
Related Questions
equator runs east and west all the way around the world,halfway between the south poles called the ______________and north poles called________________.
now na po plss....
then follow me☝
Answers
Answer:
hanigin ng mga pule natin
The amount of energy required to turn a liquid into a gas is termed.
Answers
The amount of energy required to turn a liquid into a gas is termed latent heat.
What is Latent Heat?Latent heat, also known as the heat of vaporization or latent heat of vaporization is the magnitude of heat energy necessary to change the phase of a liquid substance to gas.
The change of phase, in this case, is not accompanied by a change in the temperature or the pressure of the liquid. In other words, latent heat is the amount of heat energy required to change a liquid to a gas at constant temperature and pressure.
More on latent heat can be found here: https://brainly.com/question/20339399
What states of matter do halogens exist in at room temperature?
Answers
At room temperature, the halogens show all the states of matter.
Halogens are the elements of the periodic table that belong to group number 17 and are non-metals. Fluorine and chlorine are gaseous at room temperature, bromine exists as liquid, whereas iodine and astatine are solid in nature. The halogens are highly electronegative and reactive in nature.
States of matter are the physical form in which a substance can exist. There are three states of matter: solid, liquid and gas. Fluorine and chlorine have weak forces of attraction between their molecules at room temperature, hence gaseous. Bromine has little stronger intermolecular force that prevents its evaporation and hence a liquid. The molecular weight of higher halogens is large with strong forces molecular forces and hence are solids.
To know more about halogens, here
brainly.com/question/11156152
#SPJ1
Identify the elements that make up water and ozone .Name the element common between the two molecules .
Answers
Answer: Water is made up of elements hydrogen and oxygen. Ozone is made up of element oxygen. The common element between them is oxygen.
Explanation:
Element is a pure substance which is composed of atoms of similar elements. It can not be decomposed into simpler constituents using chemical reactions.Example: Hydrogen (H)
Water and ozone are compounds which are formed by the combination of elements in fixed ratio by mass.Water and ozone have molecular formulas of \(H_2O\) and \(O_3\) respectively.Water is made up of elements hydrogen (H) and oxygen (O). Ozone is made up of element oxygen (O).
Thus the common element between them is oxygen (O).
Number 12 plsssssssssssssssssss

Answers
Answer:
check my explanation
Explanation:
it is the secondary consumer because it doesn't eat grass
Waste water from nuclear power plants is generally:
Answers
Answer:
Nuclear energy produces radioactive waste
A major environmental concern related to nuclear power is the creation of radioactive wastes such as uranium mill tailings, spent (used) reactor fuel, and other radioactive wastes. These materials can remain radioactive and dangerous to human health for thousands of years.
A 23. 6 g sample of Na3PO4 (molar mass 163. 94 g/mol) is dissolved in enough water to produce
750. ML of solution
Calculate the concentration of Nations in solution.
Write your answer using three significant figures.
Answers
The concentration of sodium ion, Na⁺ in the solution, given that 23.6 grams of Na₃PO₄ is dissolved in 750 mL of water is 0.576 M
How to determine the concentration of sodium ion, Na⁺ ?We'll begin by obtaining the molarity of the solution. This is illustrated below:
Mass of Na₃PO₄ = 23.6 gMolar mass of Na₃PO₄ = 163.94 g/molMole of Na₃PO₄ = 23.6 / 163.94 = 0.144 moleVolume = 750 mL = 750 / 1000 = 0.75 LMolarity = ?Molarity = mole / volume
Molarity = 0.144 / 0.75
Molarity = 0.192 M
Finally we shall determine the concentration of the sodium ion, Na⁺. Details below:
Na₃PO₄(aq) <=> 3Na⁺(aq) + PO₄³⁻(aq)
From the balanced equation above,
1 mole of Na₃PO₄ contains 3 moles of Na⁺
Therefore,
0.192 M Na₃PO₄ will contain = 0.192 × 3 = 0.576 M Na⁺
Thus, the concentration of Na⁺ is 0.576 M
Learn more about molarity:
https://brainly.com/question/13386686
#SPJ1
PLEASE Answer asap! Compare two periodic families below. You must include at least two facts about each family. You may use any description of properties or periodic trends to help you.
Answers
Answer:
The alkali metals/group 1 are recognized as a group and family of elements. These elements are metals. Sodium and potassium are examples of elements in this family. Hydrogen is not considered an alkali metal because the gas does not exhibit the typical properties of the group. The largest family of elements consists of transition metals/group 3-12/group 3a. The center of the periodic table contains the transition metals, plus the two rows below the body of the table (lanthanides and actinides) are special transition metals. Even though both are metals, transition metals have higher melting points; they have higher density; they are less reactive with water; they react and form ions with different charges, but Group 1 metals only form 1+ ions. (Hope the is helpful! You can reword it if you want...)
Decide which intermolecular forces act between the molecules of each compound in the table below.
Answers
Answer
• Hydrogen bromide: dispersion and dipole-dipole
,• Bromine: dispersion
,• Hypochlorus acid : dispersion, dipole-dipole, hydrogen bond
,• Molecular oxygen: dispersion
Procedure
The London dispersion force is a temporary attractive force that results when the electrons are in two adjacent atoms. These are the weakest intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar.
Dipole-dipole: occur between polar molecules when the partially positively charged part of a molecule interacts with the partially negatively charged part of another molecule.
A hydrogen bond: is a special kind of dipole-dipole interaction that occurs specifically between a hydrogen atom bonded to an oxygen, nitrogen, or fluorine atom.
Based on the previous:
Hydrogen bromide: dispersion and dipole-dipole
Bromine: dispersion
Hypochlorus acid : dispersion, dipole-dipole, hydrogen bond
Oxygen: dispersion
Nigeria experiences several more hours of daylight in summer than in winter. Which statement helps explain this observation?
Answers
Answer:
Earth is tilted on its axis
Explanation:
Whenever Earth is tilted on its axis, the tilt of the earth's rotational axis causes Nigeria to have longer days in the summertime, beginning in June, compared to the winter period. The tilting of the earth also causes Nigeria to experience longer nights in the winter.
The earth’s axis of rotation is tilted at an angle of 23.5 degrees compared to the earth's orbital plane. Such tilting of the earth is what causes Nigeria to experience longer hours during the day in the summer when the Northern Hemisphere is titled toward the sun starting around June 20 or 21.
what is the torsional angle between c-ch 3 bonds in the totally eclipsed conformation of butane?
Answers
The torsional angle between c-ch 3 bonds in the totally eclipsed conformation of butane is 0°.Explanation:In the totally eclipsed conformation of butane,
there is a high degree of torsional strain. The eclipsed conformation has the highest energy level of all butane conformations because of the steric hindrance caused by the overlapping atoms. Atoms that overlap produce strain energy, which raises the energy of the molecule. In the butane molecule,
the maximum torsional angle for the C-C bond is 120 degrees, and the minimum torsional angle is 60 degrees.Therefore, in the totally eclipsed conformation, the C-C bond angle is 0° because the two methyl groups are eclipsed.
TO know more about that torsional visit:
https://brainly.com/question/31838400
#SPJ11
The acid-base properties of amino acids are important in their function. amino acids and proteins are both referred to as ampholytes. This means?
Answers
Ampholytes are amphoteric electrolytes. This means that they can act as both acids that is, as a proton donor, or a base as a proton acceptor.
Amino acids are made up of two ionizable groups, the positively charged amino group (NH3+) and the negatively charged carboxyl group (COOH-).
When dissolved in a neutral solution, the amino group becomes protonated, while the carboxyl group becomes dissociated. While in this state they are predominantly dipolar ions or zwitterions.
Apart from the amino and carboxyl groups, some amino acids have additional side groups that can be ionized. These include Lysine, Glutamic acids, Aspartic acid and Arginine.
To learn more about amino acids, visit:
https://brainly.com/question/15687833
#SPJ4
after the reactions are complete, suppose you have found that the reaction with 2-fluoro-5-nitropyridine was faster than the reaction with 2-bromo-5-nitropyridine. what is the most likely explanation for this observation?
Answers
The intermediate is stabilised by fluorene electronegativity, which removes the electrons from the meisenheiner complex's negative charge, allowing the process to proceed more quickly.
What is fluorene ?The chemical molecule known as fluorene, also known as 9H-fluorene, has the formula (C6H4)2CH2. It produces white crystals with a distinctive, aromatic smell akin to naphthalene. Its name comes from the violet fluorescence it emits. It is made from coal tar for commercial use.Because the Meisenheiner complex, a tetrahedral-like intermediate with a negative charge on the nitrogen of the pyridine ring, is produced by the nucleophilic attack of the benzyl amine. By stabilising the intermediate and removing the electrons from the meisenheiner complex's negative charge, fluorene electronegativity speeds up the reaction.Fluorene can cause skin and eye irritation and burns. White crystalline plates make up fluorene. It serves as a chemical intermediary as well as being employed in resinous goods and colours. Because HHAG and EPA have mentioned fluorene, it is listed on the Hazardous Substance List.To learn more about fluorene refer :
https://brainly.com/question/14764149
#SPJ4
Please help me I’ll mark you the brainiest! Please do NOT answer if you don’t know it.

Answers
Answer: It is B
Explanation:
EAZYYYY
Which actions increase the strength of an electromagnet? Question 5 options: Increase the number of coils and decrease the strength of the current. Increase the number of coils and increase the strength of the current. Decrease the number of coils and decrease the strength of the current. Decrease the number of coils and increase the strength of the current.
Answers
An electromagnet is a device in which, by electric current, the magnetic field is produced. Increase the number of coils and increase the strength of the current, thus, increasing the strength of an electromagnet.
The correct option is B.
What is an electromagnet?An electromagnet is a device that consists of a magnetic core encircled by a coil, through which an electric current is carried to magnetize the core.
Wherever controllable magnets are required, such as in devices where the magnetic flux must be adjusted, reversed, or switched on and off, an electromagnet is utilized.
Thus, the correct option is B, increasing the number of coils and increasing the strength of the current.
Learn more about electromagnet, here:
https://brainly.com/question/13803241
What is the relation between amino acids and polypeptide?
Answers
The relation between amino acids and polypeptide is that amino acid is monomer and polypeptide is polymer.
A member of the class of organic molecules known as amino acids, each of which is made up of a special organic R group, an acidic carboxyl group (COOH), a basic amino group (NH2), and an amino group (NH2) (or side chain). Alpha-amino [alpha-amino] carboxylic acid is the colloquial term for an amino acid. Every molecule has a central carbon (C), also known as the -carbon, that is attached to an amino group and a carboxyl group. Typically, a hydrogen (H) atom and a R group are used to fill the final two bonds of the -carbon atom. Standard (or common) amino acids can be divided into groups according to the polarity (i.e., the distribution of electric charge) of the R group (e.g., side chain).
To know more about amino acids visit : https://brainly.com/question/4686866
#SPJ4
Calculate the mass of sample of Palladium in lbs if the heat of fusion of Pd is 162J/g and it takes 686J of energy to melt the sample.
Answers
The copper cools from 100 °C to 0
The aluminium and spirit both warm up from 10.0 °C to 0 Proceeding as in the previous examples, remembering to work in kg,
Heat in J given out by copper
Heat in J received by aluminium
Heat in J received by spirit
= 0.25 x 400 x (1000)
= 0.01 × 900 x (0-10)
= 0.12 × 2400 × (0 10)
Heat given out = heat received
therefore
100 (1000) = 9 × (010) + 288 × (0 10)
or,
10 000 100 0 297 0 2970 - =
12 970 397 0
12970
0 =
=
12.970
397
= 32.7 °C
Rearranging,
whence
help me please, with both number 2 and 3, first one to answer gets a brainliest!

Answers
Answer:
2) a
3) c
explanation:
both gain from the relationship in number 2
while in number 3 one gains and one is hurt
if two objects have the same density, they must have the same
Answers
Answer:
no ,its not necessary tht two objects have same volume have the same mass because the density of the material they are made up of can be different. mass and volume areindependent, two objects with thesame volume can have differentmasses. Therefore, the objects can have different densities.
Two samples of sodium chloride were decomposed into their constituent elements. One sample produced 2.84 g of sodium and 4.37 g of chlorine. Which of the following could be the results of the decomposition of the other sample, being consistent with the law of constant composition (also called the law of definite proportions or law of definite composition)?
a) 4.17 g of sodium and 3.75 g of chlorine
b) 4.17 g of sodium and 6.42 g of chlorine
c) 4.17 g of sodium and 1.05 g of chlorine
d) 4.17 g of sodium and 12.1 g of chlorine
Answers
Answer:
The correct answer is b) 4.17 g of sodium and 6.42 g of chlorine
Explanation:
According to the law of definite proportions a chemical compound is composed always by the same elements in the same proportions by mass. In this case, the proportion of the elements by mass will be 4.37 g of chlorine (Cl) per 2.84 g of sodium (Na):
4.37 Cl/2.84 g Na= 1.54
We can calculate the proportions of the results in order to see which is the correct:
a) 3.75 g Cl/4.17 g Na = 0.899
b) 6.42 g Cl/4.17 g Na = 1.539 ⇒ ≅1.54
c) 1.05 g Cl/4.17 g Na = 3.971
d) 12.1 g Cl/4.17 g Na = 2.901
The option in which the proportion Cl/Na is equal to 1.54 is option b
If an atom of element X bonds with another atom of X, will the bond between the atoms be ionic, polar covalent, or non polar covalent?
Answers
Explanation:
If an element X bonds with another atom of X then the bonds between the atoms will be non-polar covalent bond.
Identify the choice that best completes the statement or answers the question. The purpose of a car engine is to transform the chemical energy of gasoline into kinetic energy of the car in motion. Gasoline is burned in the engine to create that movement. However, gasoline engines are typically only about 20% efficient. What happens to the rest of the energy released from the burning gasoline? Most of it is destroyed by the process of the gasoline burning. Most of it is changed through chemical reaction to a type of nuclear energy. Most of it is transformed by the engine into electrical energy. Most of it is transferred to the environment in the form of heat.
Answers
Answer: Most of it is transferred to the environment in the form of heat.
Explanation:
For every system, the law of conservation of energy is applicable which states that the energy of the system remains conserved. Energy can neither be created nor destroyed.
When gasoline is burnt, some of the chemical energy stored in the chemical bonds of gasoline is converted to kinetic energy which is used to drive the vehicle and some of it is lost in the form of heat energy to the surroundings. But the total energy remains conserved.
If a system loses energy, an equivalent amount of energy is gained by surroundings, thus the total energy remains constant.
Answer:
Answer: Most of it is transferred to the environment in the form of heat.
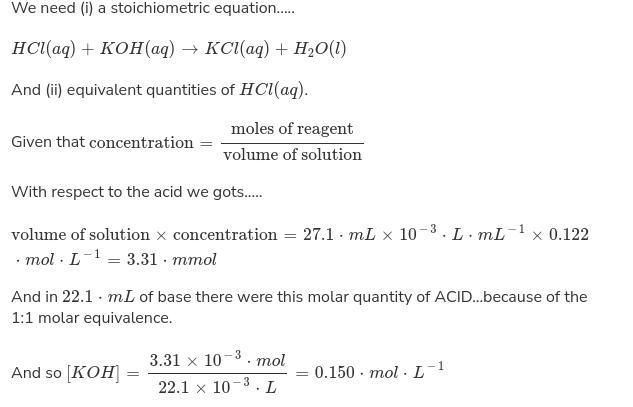
An aqueous solution of potassium hydroxide is standardized by titration with a 0.167 M solution of nitric acid. If 16.6 mL of base are required to neutralize 27.7 mL of the acid, what is the molarity of the potassium hydroxide solution
Answers
The molarity of the potassium hydroxide solution is 0.150 mol/L.
Molar attention is a measure of the attention of a chemical species, in particular of a solute in an answer, in phrases of quantity of substance consistent with the unit extent of solution.
Molarity (M) is the amount of a substance in a sure volume of answer. Molarity is defined as the moles of a solute according to the liters of a solution. Molarity is also referred to as the molar attention of a solution.
Molarity is the wide variety of moles of solute consistent with the liter of answer. For example, in case you dissolve desk salt in water, salt is the solute, and water is the solution. One mole of sodium chloride weighs 58.44 grams. in case you dissolve 58.44 grams of NaCl in a single liter of water, you have got a one molar solution, abbreviated as 1M.
Learn more about molarity here https://brainly.com/question/14469428
#SPJ4
7
When considering an argument's reasoning, it's important
to be:
А
Optimistic
B
Trusting
C
Quick
D
Skeptical
Answers
Answer:b
Explanation:because people have to believe you
Help me plssssssssssssssssssssssssssssss

Answers
Answer:
About half way through its fall
Explanation:
Firstly, let's talk about the thermal energy - we can pretty much disregard it because of how miniscule it is compared to the rest and because it's not VERY connected to the process of falling down.
m - mass
v - velocity
g - gravitational acceleration constant (~9.81m/s^2)
Ek - kinetic energy
Ep - potential energy
The kinetic energy is dependent on the velocity - the faster an object moves, the bigger its kinetic energy. In the classic model Ek = mv^2/2
The potential energy is dependent on the altitude - the higher the object is located, the longer it'll be able to fall down. In the classic model Ep = mhg
The process of falling converts the potential energy to kinetic energy - notice that as something falls down, the altitude is rapidly reduced (and so is the potential energy) but the velocity is rising (and so the kinetic energy).
When the ball is in your hand, it's static, so v = 0, so the kinetic energy is
Ek = mv^2/2 = m*0^2/2 = 0.
When the ball hits the ground, it's altitude is 0, so its potential energy is
Ep = mhg = m*0*g = 0.
When it has stopped moving at all, again its v = 0, so the kinetic energy is 0.
Mid fall, it's still got a ways to go, so its potential energy is positive, it also has some velocity, so its kinetic energy is also positive. "About halfway through the fall" is a very acceptable answer here.
Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
Temperature, according to the kinetic molecular theory, is a measure of intermolecular attraction.
TRUE or FALSE
Answers
what four elements does halogen bond with?
Answers
fluorine, chlorine, bromine, and iodine,
HELPPPPPPPPPPPPPPPPPPPPPPPPPPPP

Answers
Answer:
the awnser is a
Explanation:
obvious lab safetty
PLS HELP USING ALL MY POINTS AND WILL MARK BRAINLIEST
1. Fill in table 5.2
2. Which metal is the most reactive? How do you know this?
3. Rank the metals in order of increasing reactivity
4. Give the chemical equations for each single replacement reaction that took place
5. Was Fe^3+ reduced? If so, what metal(s) acted as reducing agents?

Answers
Answer:
54.09
Explanation: