Answers
Answer:
The limiting reagent is O2. The theoretical yield of N2O4 is 6 moles.
The limiting reactant is nitrogen.
In a chemical reaction, two or more species combine to form products. The amount of all the reactants in the system are usually not the same. The specie that is present in the least amount is called the limiting reactant. The reactant that is present in the greatest amount is called the reactant in excess.
Looking at the image, there are six oxygen molecules and two nitrogen molecules hence nitrogen is the limiting reactant and oxygen is the reactant in excess.
Since the equation of the reaction is; 2N2 + 4O2 --->2N2O4, it follows that there are two excess oxygen molecules that are left unreacted. Two N2O4 molecules are formed.
Learn more:https://brainly.com/question/17762711
Missing parts:
The illustration to the left represents a mixture of nitrogen (blue) and oxygen (red) molecules.
If the molecules in the above illustration react to form N2O4 according to the equation N2 + 2O2 N2O4
the limiting reagent is___?___
the number of N2O4 molecules formed is___?___
the number of___?___molecules in excess is___?___

Related Questions
why does the ratio of chloride ions to calcium ions is 2:1 when calcium chloride forms
Answers
An Ibuprofen (MW = 206.29) solution was made by dissolving 4,421 milligrams into water with a
final volume of 0.172 L. What is the concentration of Ibuprofen in Molarity?
Answers
The concentration of Ibuprofen in the solution is approximately 0.124 M (Molarity), calculated by dividing the moles of Ibuprofen (0.0214 mol) by the volume of the solution (0.172 L).
To calculate the concentration of Ibuprofen in molarity, we need to use the formula:
Concentration (Molarity) = moles of solute / volume of solution in liters
First, we need to convert the mass of Ibuprofen into moles. The molar mass of Ibuprofen is given as 206.29 g/mol.
Step 1: Convert milligrams to grams:
4,421 mg = 4.421 g
Step 2: Convert grams to moles:
moles = mass / molar mass
moles = 4.421 g / 206.29 g/mol
moles ≈ 0.0214 mol
Step 3: Calculate the concentration:
Concentration = moles / volume
Concentration = 0.0214 mol / 0.172 L
Concentration ≈ 0.124 M
Therefore, the concentration of Ibuprofen in the solution is approximately 0.124 M (Molarity). This means that there are 0.124 moles of Ibuprofen dissolved in every liter of the solution.
Know more about Molarity here:
https://brainly.com/question/30404105
#SPJ8
draw a potentail energy diagram for a combustion reaction
Answers
Potential energy diagram for a combustion reaction:. CLICK ON IMAGE.
In a typical combustion reaction, the reactants (e.g. a fuel and an oxidizer) are initially at a relatively high potential energy. As the reaction proceeds, the potential energy of the system decreases, and the products (e.g. carbon dioxide and water vapor) are at a lower potential energy than the reactants. The difference in potential energy between the reactants and products corresponds to the heat released during the reaction.
The diagram shows the initial energy level of the reactants, the activation energy required to initiate the reaction, and the final energy level of the products. The activation energy is the minimum amount of energy required for the reaction to occur.
Once the reactants have absorbed enough energy to reach the activation energy threshold, the reaction proceeds spontaneously and releases energy as it progresses to the lower-energy products.
Note that the shape of the potential energy diagram can vary depending on the specific reaction and the reaction conditions. For example, some reactions may have more complex energy profiles with multiple intermediate steps or energy barriers.
For more question on Potential energy click on
https://brainly.com/question/18963960
#SPJ11

Chlorofluorocarbons are ?
A. colorless, odorless gases that prevent red blood cells from carrying oxygen to the body
B. man-made chemicals containing chlorine and fluorine that cause
ozone molecules to break down
C. chemicals produced in factories that are used to prevent air
pollution
D. molecules containing chlorine and fluorine that block UV radiation
from reaching the Earth
Answers
Chlorofluorocarbons (CFCs) are synthetic compounds that contain chlorine, fluorine, and carbon. They were widely used in the past as refrigerants, propellants in aerosol products, and foam-blowing agents. CFCs have been found to have a detrimental effect on the Earth's ozone layer when released into the atmosphere. They can reach the stratosphere, where they undergo a chemical reaction facilitated by ultraviolet (UV) radiation, resulting in the release of chlorine atoms. These chlorine atoms then participate in a destructive cycle that breaks down ozone molecules, leading to ozone depletion. Due to their harmful impact on the ozone layer, the production and use of CFCs have been phased out or regulated under international agreements like the Montreal Protocol to protect the Earth's ozone layer.
Chlorofluorocarbons (CFCs) are man-made chemicals containing chlorine and fluorine that cause ozone molecules to break down. Thus, option B is the answer.
Chlorofluorocarbons are non-toxic, synthetic compounds that contain atoms of Chlorine, Fluorine and Carbon. They are commonly used in the manufacture of aerosol sprays and are also used as solvents and refrigerants. CFCs were first introduced in 1928 by General Motors Company for its refrigerators.
While CFCs are very safe to use in most applications and are stable in the lower atmosphere, these chemicals when released to the upper atmosphere can cause significant reactions. CFCs when released into the upper atmosphere can lead to the destruction of the ozone molecules followed by the release of the UV radiation into the atmosphere.
Thus, CFCs are man-made chemicals which cause ozone molecules to break down.
Learn more Chlorofluorocarbons, here:
https://brainly.com/question/1393491
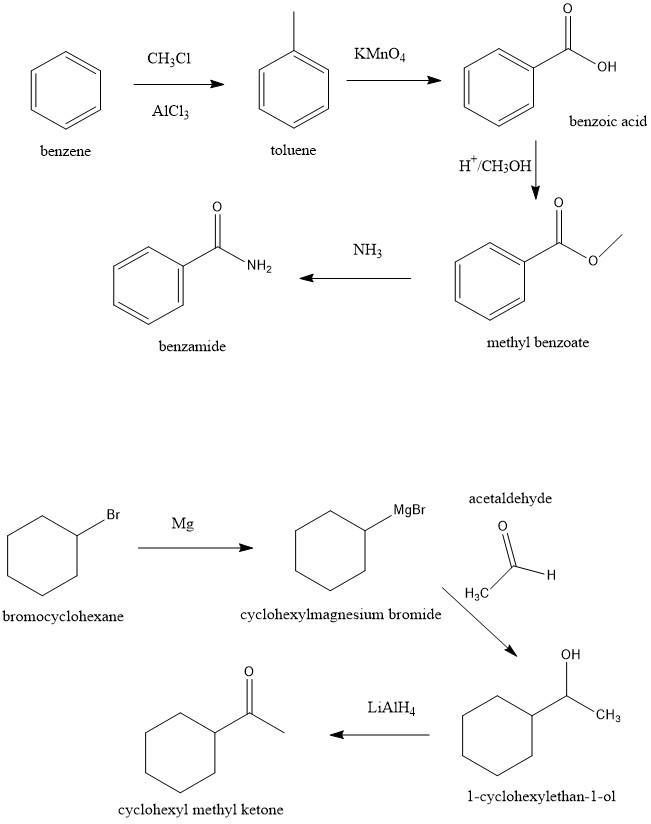
10. Show as many ways as you can think of: a) to make benzamide from benzene; b) to make cyclohexyl methyl ketone from bromocyclohexane;
Answers
Answer:
See explanation
Explanation:
a) Benzamide from benzene
For this synthesis, we have to start with the Friedel-Crafts reaction to produce Toluene. Then with a strong oxidant, we can produce benzoic acid. In the next step, we can use an esterification reaction to produce the methyl benzoate. Finally, we can use an acyl substitution reaction using ammonia to produce the benzamide.
b) From bromocyclohexane to cyclohexyl methyl ketone
In this case, we can start with a Grignard reaction. The first step is to produce the Grignard reagent with using magnesium. Then if we add acetaldehyde we can form an alcohol, 1-cyclohexylethan-1-ol. Finally, we can reduce the alcohol to produce cyclohexyl methyl ketone.
See figure 1
I hope it helps!
Could someone draw me electron dot diagrams? The Hydride Ion, Hydrogen Ion, Aluminum Ion, Nitride Ion, Oxide Ion, and Calcium Ion, and Sodium-Ion dot structures? Thanks!
Answers
Answer:
urorrr
Explanation:
hahhahahahahhaahah oki nam omy mymy mymy
For the reaction 2 Kl+Pb(NO 3 ) 2 Pbl 2 +2 KNO 3 how many grams of lead(II) nitrate, Pb(NO 3 ) 2 are needed to react completely with 71.9 g of potassium iodide, KI?
Answers
71.73grams
Explanations:Given the chemical reaction;
\(2KI+Pb(NO_3)_2\rightarrow PbI_2+2KNO_3\)Given the following parameter
Mass of KI = 71.9grams
Determine the moles of KI
According to stoichiometry, 2moles of KI reacts with 1 mole of lead(II) nitrate,the moles of lead(II) nitrate that reacted is given as:
\(\begin{gathered} moles\text{ of Pb\lparen NO}_3\text{\rparen}_2=\frac{1}{2}\times0.4331 \\ moles\text{ of Pb\lparen NO}_3\text{\rparen}_2=0.2166moles \end{gathered}\)Determine the mass of lead(II) nitrate
\(\begin{gathered} mass\text{ of Pb\lparen NO}_3\text{\rparen}_2=moles\times molar\text{ mass} \\ mass\text{ of Pb\lparen NO}_3\text{\rparen}_2=0.2166\times331.2 \\ mass\text{ of Pb\lparen NO}_3\text{\rparen}_2=71.73g \end{gathered}\)Hence the mass of lead(II)nitrate needed to react completely with 71.9 g of potassium iodide is 71.73grams
Is salt more or less dense than water?
Answers
Answer:
less dense
Explanation:
because when u put it in it sinks
Answer:
salt is more denser than water.
Explanation:
it makes the mass of water denser.
172 °C = __________ K
(Round off to the nearest whole number)
Answers
Nitrogen is a group 15 element. What does being in this group imply about the structure of the nitrogen atom?
O A. Nitrogen has 15 valence electrons.
OB.
Nitrogen has 15 neutrons.
OC. Nitrogen has 5 valence electrons.
D.
Nitrogen has 5 neutrons.
Answers
Answer:
D. Nitrogen has 5 valence electrons.
Explanation:
Nitrogen is an element in group 5A of the periodic table. Elements in group 5A all contain just 5 valence electrons. (Electrons in the outer shell).
**Elements are organized into these groups in a periodic table based on the number of valence electrons which determines their charge. (Does not apply to transition metals)
list three common uses of copper and three common uses of silver
Answers
Silver: utensils, dishes, and rings.
Why is molecular polarity important for life?
Answers
Molecular polarity is important for life because it plays a crucial role in many biological processes.
What is molecular polarity?Molecular polarity refers to the distribution of electrical charge within a molecule. It is a property that results from differences in the electronegativity of the atoms in a molecule.
Molecular polarity is important for life because many biological molecules, including proteins, DNA, and carbohydrates, are polar, meaning they have regions of positive and negative charge.
This allows them to interact with other polar molecules, such as water, through hydrogen bonding, which helps to stabilize their structures and maintain their functionalities.
More on molecular polarity can be found here: https://brainly.com/question/4248472
#SPJ1
Determine E° for a galvanic (voltaic) cell if ∆G° = -6.3 kJ/mol and n = 3. (F = 96,500 J/(V・mol))
Answers
The E° for a galvanic cell is 0.000217 volts if ∆G° = -6.3 kJ/mol and n = 3. (F = 96,500 J/(V・mol).
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
E°=ΔG°/-nF= -6.3/-3×96500=0.000217 V.
Learn more about galvanic cell,here:
https://brainly.com/question/30268944
#SPJ1
Two stereoisomers are obtained from the reaction of HBr with (S)-4-bromo-1-pentene. One of the stereoisomers is optically active, and the other is not. Draw the structure of the optically active stereoisomer.
Answers
Answer:
See explanation
Explanation:
In this case, we have an addition reaction. Additionally, this is a marknovnikov addition, therefore the "Br" atom would be added in the most substituted carbon (in this case carbon a). And we are going to have 2 enantiomers (2S,4S)-2,4-dibromopentane and (2R,4S)-2,4-dibromopentane. In the case of (2R,4S)-2,4-dibromopentane we will have a symmetry plane (a point in the molecule in which we can divide the molecule into two equal parts). When this happens we will have a mesocompound and we will not have optical activity.
See figure 1
I hope it helps!

The density of molasses is 2.28g/mL. What volume of molasses would have a mass of 392.8g?
Answers
Answer:
V = 172.28 mL
Explanation:
density is mass divided by volume
p = density
m = mass
V = volume
p = m/v
V = m/p
V = 392.8/2.28
V = 172.28 mL
Nitrogen and hydrogen react to form ammonia, like this:N2(g)+3H2(g)→2NH3(g)Use this chemical equation to answer the questions below.Suppose 135, mmol of N₂ and 405, mmol of H₂ are added to an empty flask, How much N₂ will be in the flask at equilibrium? a. Noneb. Some, but less than 135, mmol.c. 135,mmold. More than 135, mmol.Suppose 235, mmol of NH₃ are added to an empty flask, How much N₂ will be in the flask at equilibrium? a. Noneb. Some, but less than 118, mmol.c. 118,mmold. More than 118, mmol.
Answers
Answer:
Option A is correct, there will be no N2 left in the flask
Explanation:
Step 1 : Data given
Number of moles of N2 = 135 mmol = 0.135 mol
Number of moles of H2 = 405 mmol = 0.405 mol
Step 2: The reaction
N2(g)+3H2(g)→2NH3(g)
Step 3:
For 1 mol N2 we need 3 moles H2 to produce 2 moles NH3
Both will completely react. There is no limiting reactant.
There will be produce 0.270 moles NH3.
Option A is correct, there will be no N2 left in the flask
When a conditions could exist: liquid is in dynamic equilibrium with its vapor at a given temperature, the following
(I) There is no transfer of molecules between liquid and vapor
(II) The vapor pressure has a unique value
(III) The opposing processes, (liquid to vapor) and (vapor to liquid), proceed at equal rates
(IV) The concentration of vapor is dependent on time
Which of the above choices are applicable?
a. I
b. II and III
c. I, II, and III
d. II and IV
e. none of these combination
Answers
In dynamic equilibrium conditions, the vapor pressure is unique, and the rate of vaporization and condensation are at equal rates. Thus, option b is accurate.
What is dynamic equilibrium?A dynamic equilibrium is a reaction state where the rate of the forward and the backward reaction are equal. In the above case, the vapourization and the condensation will occur simultaneously at the same rate.
The vapor pressure of the liquid to gas and vice versa has a distinctive value as the temperatures are different making the pressure change directly.
Therefore, option b. II and III are the correct options.
Learn more about dynamic equilibrium here:
https://brainly.com/question/17354479
#SPJ1
I
II
III
none of the above

Answers
Answer:
i think it will be the first one
Explanation:
At standard conditions 3.56L of a gas weighed 11.3 g. Its molar mass isGroup of answer choices3.17 g/mole11.3 g/mole40.2 g/mole71.1 g/mole79.7 g/mole
Answers
Explanation:
The molar volume corresponds to the volume occupied by any gas at STP, which is equal to 22.4 L.
So:
22.4 L ---- 1 mol
3.56 L ---- x mol
x = 0.159 moles
To find the molar mass we use the following formula:
molar mass = mass/moles
molar mass = 11.3/0.159
molar mass = 71.1
Answer: 71.1 g/mole
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
Complete the fourth column of the table.Express your answer using two significant figure

Answers
Using Avogadro's Law, we have
\(\frac{V_1}{n_1}=\frac{V_2}{n_2}\)In the fourth column, we have to find n2.
\(n_2=\frac{V_2\cdot n_1}{V_1}\)Where V1 = 8.66 L, V2 = 10.9 L, and n1 = 0.0021 mol.
\(n_2=\frac{10.9L\cdot0.0021\text{mol}}{8.66L}=0.0026mol\)Therefore, n2 = 0.0026 moles.
6.) (5 points) Assume you have a system with a fixed pH of 4.0. It is well buffered and therefore the pH will not change. What is the predominant state of a molecule with a dissociable proton with a pKa of 5.2 if it is introduced to that system (protonated or deprotonated)
Answers
Answer:
Dissociated state is the predominant one
Explanation:
When a molecule with pKa of 4.52 is in an aqueous solution at pH = 4.0, follows the H-H equation, thus:
pH = pKa + log₁₀ [A⁻] / [HA]
Where [A⁻] is the dissociated state and [HA] represents the protonated state
Replacing:
4.0 = 5.2 + log₁₀ [A⁻] / [HA]
-1.2 = log₁₀ [A⁻] / [HA]
0.063 = [A⁻] / [HA]
[HA] = 16 [A⁻]
That means you have 16 times more [HA] than [A⁻] and the dissociated state is the predominant one
Look at the diagram.
Which two structures are first to combine in translation?
1 and 4
2 and 3
3 and 4
1 and 2

Answers
The two structures are first to combine in translation are 3 and 4. Therefore, option C is correct.
What is translation ?The process in which the information encoded in the mRNA is used to direct the sequencing of amino acids, and therefore it finally synthesizes a protein is called as translation.
In translation the information passed from DNA to mRNA and turns it into a series of amino acids bound together with peptide bonds.
Translation is translation from one code of nucleotide sequence to another code of amino acid sequence.The two structures are first to combine in translation are 3 and 4.
Thus, option C is correct.
To learn more about translation, follow the link;
https://brainly.com/question/12463306
#SPJ1
what element is 1s2 2s2 2p6 3s2 3p6 3d7 4s1
Answers
Answer:
The element is Iron
Explanation:
gold flakes extracted thru the head/hair with over exposure limits inhalation of methylene chloride, how could this happen, chlorination extraction of gold from the body. it happen
Answers
Chlorination extraction of gold from the body may refer to the use of methylene chloride to extract gold particles from the scalp and hair. During prolonged exposure, the methylene chloride can be inhaled which can cause serious health issues.
What is Chlorination extraction of gold?This method was founded on the idea that gold turns into the trichloride AuCl₃ when chlorine reacts with moisture. This compound can then be washed away, and the gold is precipitated using ferrous sulfate, sulphate of potash, hydrogen sulfide, or charcoal. Long contact is necessary to remove coarse gold, which should be done via amalgamation. Thoroughly oxidized ore may be handled immediately for pyrite ore or concentrate, but basic ores—which contain lime and particularly magnesia—absorb a lot of chlorine and may become heated. This was verified by finishing the roasting at a high temperature to frit the magnesia with silica.
What is Amalgamation?A merger unites two or more businesses to form a new organization. A merger and an amalgamation are not the same thing because neither business exists as a separate legal entity. Instead, a brand-new organization is created to hold the merged assets and obligations of the two businesses.
Even when a new company is formed, the term amalgamation has typically lost favor in the United States and been replaced with merger or consolidation.
To know more about methylene chloride, visit:
https://brainly.com/question/29426391
#SPJ1
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
the difference between Major purchase Consumer good
Answers
A major purchase refers to a high-cost item or service that is considered a significant investment for an individual or household, such as a car, home, or college education. Major purchases usually involve a large sum of money and require careful planning and consideration before a final decision is made.
Any item that a person or a household buys for their own use or consumption is called a consumer good. Durable and non-durable goods are two types of consumer goods that can be classified. Consumer goods are tangible items that people or households buy for their own use or consumption.
Learn more about major purchase, here:
https://brainly.com/question/29998590
#SPJ1
Your question is incomplete, most probably the complete question is:
The difference between Major purchase and Consumer good?
Some students want to investigate the relationship between the pressure and volume of a gas. They take a sealed, thin-walled metal can full of air, place it in a vat of liquid nitrogen, and observe that the can collapses. Another student says that their procedure is a better demonstration of the effect of temperature on another property of the gas. Which of the following properties of the gas does the procedure best relate to temperature?
a. Volume.
b. Pressure.
c. The amount of gas.
d. The universal gas constant.
Answers
The ideal gas equation allows finding that the best variable for the temperature experiment is:
a. Pressure
b. Volume
Ideal gases are gases that do not have any interaction between their molecules, they are described by the relationship
P V = n R T
Where P is the pressure, V the volume, n the number of moles, R the ideal gas constant and T the absolute temperature.
In the problem the students want to study the effect of temperature with ideal gases equation we see that pressure and volume are directly proportional to temperature.
Of these two properties, the easiest to measure is pressure, keeping the volume constant.
In conclusion using the ideal gas equations allows finding that the best variable for the temperature experiment are :
a. Pressure
b. volume
Learn more here: brainly.com/question/3491421
What color does red cabbage juice make when mixed with a citrus cleaner?
What color does red cabbage juice make when mixed with a dishwasher soap?
(WILL MARK BRAINLIEST!!!)
Answers
Answer:
Explanation:
green
What is the number of moles in 0.025g (NH4)2Cr2O7
Answers
Answer:
9.91 X 10^-5 or 0.000099 mol
Explanation: