“How does gas volume change with pressure?”
CHEMISTRY HW!!!!
WILL GIVE “THANKS” & A BRAINIST

Answers
Hope this helps
Have a great day! Good day! :)
Related Questions
What is the outcome of all chemical changes when two substances are combined?
A. A new substance forms with identical properties.
B. The two substances release an odor.
C. The two substances change color.
D. A new substance forms with different properties.
Answers
Answer:
D
Explanation:
Because when you combine chemical substances they create a new substance unlike physical substances .
Answer:
D- A new substance forms with different properties
Explanation:
When a chemical change occurs, it causes the matter to form into a completely new substance. Because of this, the new substance will have different properties than the original substance. Options B and C are true for some chemical changes, but not all changes. Option A is incorrect because the substance has different properties than the original substance. Thus, Option D is correct.
- I took the quiz and got 100%
Hope this helps!

The freezing point of a substance is the
at
which it freezes.
Answers
Answer:
reff lagay mo yung ilalagay mo
Answer:
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure. Alternatively, a melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
I need help with this question.
Instruction : Answer the following questions using the given options.
Questions :
1. The reaction between an acid and a base is an example of ___ reaction ?
a. Valence
b. Endothermic reaction
c. Chemical reaction
d. Exothermic reaction
2.The following are the factors affecting the rate of a chemical reaction except ___
a. catalyst
b. nature of reactants
c. activation energy
d. lights
3.___ gives a dense white fume when in contact with HCL
a. propane
b. ammonia
c. tetraoxosulphate(vi) acid d. water
Answers
Explanation:
The reaction between an acid and a base is known as a neutralisation reaction.
Reactant concentration. Increasing the concentration of one or more reactants will often increase the rate of reaction. ...
Physical state of the reactants and surface area. ...
Temperature. ...
Presence of a catalyst.
ammonia
An alkaline gas which produces dense white fumes when reacted with HCl gas is ammonia and fumes are of compound ammonium chloride.
BRAINILIEST PLEASE
Summarize how the structure of organic compounds allows them to function as pigments in 2 – 3 sentences
Answers
The structure of organic compounds allows them to function as pigments through chromophore and acid or basic groups.
What is a Pigment?This is defined as a colored substance which is completely or nearly insoluble in water.
The structure of organic compounds allows them to function as pigments include chromophore and the acid or basic groups such as OH, SO3H, etc.
Read more about Pigment here vhttps://brainly.com/question/1056549
#SPJ1
Why would it be harder to jump on the planet Jupiter than on Earth?
HELP PLSSS

Answers
what particle matters most to get a stable atom
Answers
A black mineral is really shiny but you not sure if its a metallic or non-metallic luster but it leaves a white to very pale gray streak, is barely able to scratch glass, you're not sure it it has cleavage or not but there are some small flat faces, looks splintery (like wood grain) is -biotite -calcium plagioclase feldspar -augite -potassium feldspar (K-spar_ -sodium plagioclase feldspar -hornblende -quartz -muscovite
Answers
Among the given options, muscovite is the best match for the described mineral characteristics.
Based on the given observations, the mineral that fits the description is "muscovite." Here's why:
Metallic or non-metallic luster: Muscovite typically exhibits a non-metallic luster. It appears shiny, but without a metallic reflection.
Streak color: Muscovite has a white to very pale gray streak, which matches the description provided.
Hardness: Muscovite has a hardness of around 2.5 to 3 on the Mohs scale, which means it is barely able to scratch glass.
Cleavage: Muscovite has excellent basal cleavage, which means it tends to break along flat, thin sheets or layers.
Splintery appearance: Muscovite often displays a splintery or micaceous appearance due to its characteristic sheet-like structure, resembling wood grain.
To know more about minerals, refer:
https://brainly.com/question/26705337
#SPJ4
Which of the following reactions in glycolysis is an aldose to ketose isomerization?
A) Enolase
B) Phosphoglycerate mutase
C) Phosphohexose isomerase
D) Aldolase
E) Glyceraldehyde-3-phosphate dehydrogenase
Answers
C) The reaction in glycolysis that involves an aldose to ketose isomerization is option Phosphohexose isomerase. Phosphohexose isomerase catalyzes the conversion of glucose-6-phosphate (an aldose) to fructose-6-phosphate (a ketose).
The reaction in glycolysis that involves an aldose to ketose isomerization is catalyzed by phosphohexose isomerase. This enzyme converts glucose-6-phosphate, which is an aldose sugar, into fructose-6-phosphate, which is a ketose sugar.
This is a crucial step in glycolysis as it allows for the rearrangement of the sugar molecule, facilitating further downstream metabolic processes.
The isomerization reaction occurs through the shifting of functional groups within the sugar molecule, resulting in the conversion from an aldose to a ketose.
This isomerization step ensures the proper progression of glycolysis and the generation of energy in the form of ATP.
To learn more about glycolysis here
https://brainly.com/question/30461189
#SPJ4
what is the role of oxygen in energy yielding pathways
Answers
Oxygen plays a crucial role in energy-yielding pathways by serving as the final electron acceptor in the electron transport chain (ETC) during cellular respiration.
Oxygen is the most important factor in energy-yielding pathways. The oxygen molecule is the final acceptor of electrons in cellular respiration, which is the process of energy production in cells. When electrons are passed down the electron transport chain, they lose energy, which is then used to pump hydrogen ions (protons) out of the mitochondrial matrix. This creates a concentration gradient of hydrogen ions, which then flow back into the matrix through ATP synthase.
The flow of hydrogen ions back into the matrix releases energy that is used to produce ATP from ADP and inorganic phosphate. Oxygen, as the final electron acceptor, is essential for this process because it helps to maintain the electron transport chain by accepting the electrons at the end of the process and allowing the cycle to continue. In summary, oxygen's role in energy-yielding pathways is crucial for the production of ATP, the main source of energy for cellular processes.
Learn more about cellular respiration here:
https://brainly.com/question/32872970
#SPJ11
Can someone please help me!!
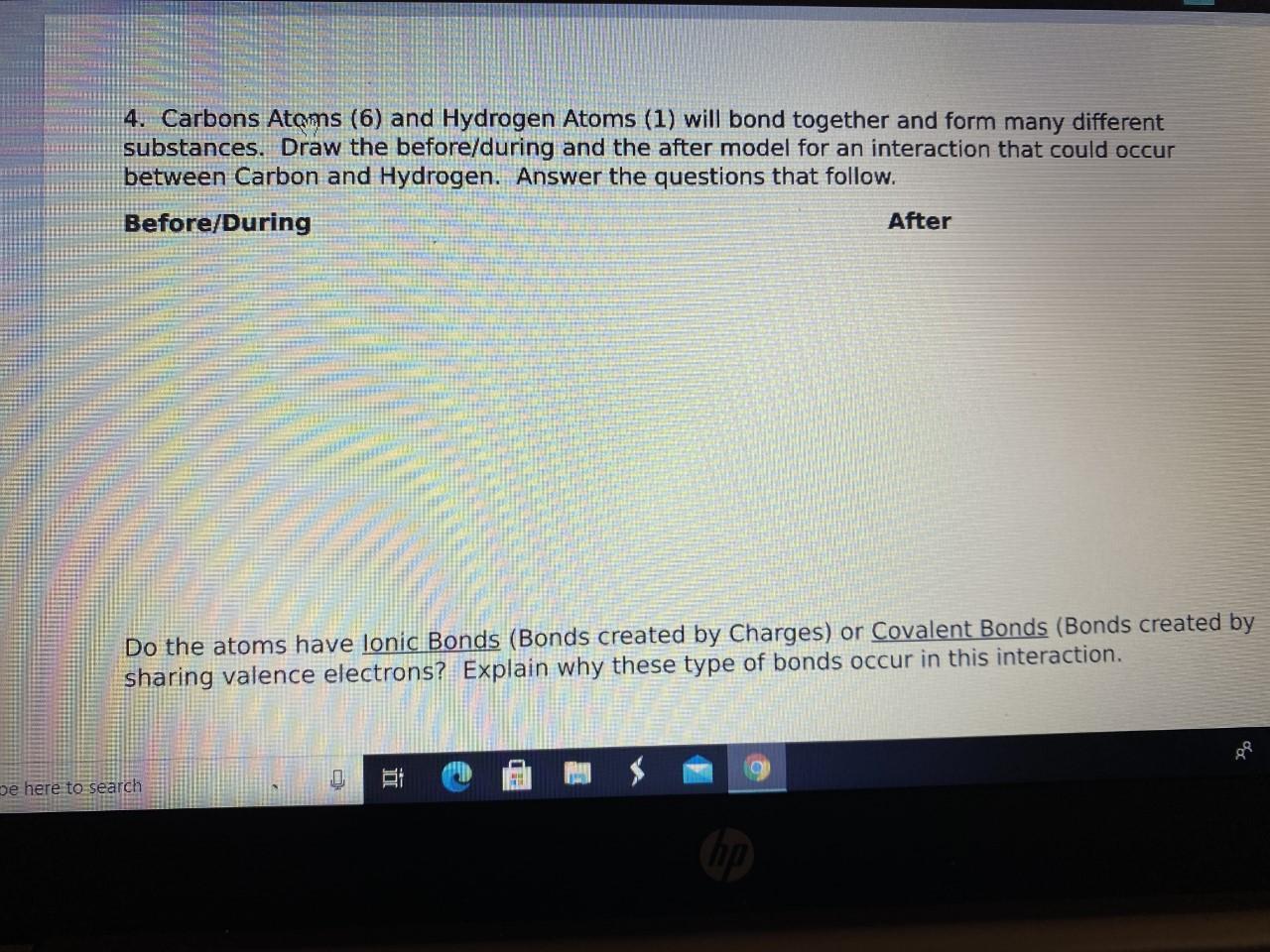
Answers
Answer:
Explanation:
Hope this helps u!!


If you added a big pile of rocks into the boat so that the mass of the rocks was greater than the displaced water, what would happen to the ship?
Answers
Answer:
it would sink to the bottom of the ocean and break in half
Explanation:
Explanation:
so if the ship was on displaced water then the ship would sink to the bottom of the ocean
Question 2 (1 point)
Which state of matter has the lowest density and the highest energy?
Answers
Hope this helps! ☺️
What is the percent sodium in sodium chloride?
Answers
The total mass of sodium chloride is 58.44 g/mol.
The mass of sodium is 22.99 g/mol.
To find the percent sodium in sodium chloride can be found by dividing the amounts.
\(\frac{22.99}{58.44}\approx0.39\)Therefore, the percent sodium is 39%.
A gas occupies a volume of 10 liters at a pressure of 0.5 atm. What is the pressure if the volume increases to 25.0 L? *
A.0.4 atm
B.0.3 atm
C.0.2 atm
D.0.1 atm
Answers
Answer:
0.2atm
Explanation:
By Boyle's law
P1V1=P2V2
0.5 x 10 = P2 x 25
therefore, P2= 0.2atm
5. If you combine 15.6 g Mg with 13.9 g of Br, what is the percent composition of each
element in the mixture.
Answers
Answer:
Mg: 52.9%
Br: 47.1%
Explanation:
Which statement is a testable hypothesis?
A. Beans are the best plants to use in a science fair experiment.
B. Watering bean plants with salt water will make them grow faster.
C. Bean plants will become extinct before corn plants do.
D. Everyone can grow beans given the right instructions.
please help. i’ll give brainliest if ur answer is correct
Answers
Answer:
b
Explanation:
I think B because its saying that perhaps watering with salt water would make them grow faster, you don't know yet unless tested.
bereman, m. s., maclean, b., tomazela, d. m., liebler, d. c., and maccoss, m. j. (2012) the development of selected reaction monitoring
Answers
The given text appears to be a citation or reference for a scientific paper titled "The Development of Selected Reaction Monitoring" by Bereman, M. S., Maclean, B., Tomazela, D. M., Liebler, D. C., and MacCoss, M. J. published in 2012.
Selected Reaction Monitoring (SRM) is a targeted mass spectrometry technique used for quantitative analysis in proteomics research. It is commonly employed to measure specific peptides or proteins in a complex biological sample. SRM allows for the detection and quantification of molecules of interest with high sensitivity and specificity.
In SRM, a mass spectrometer is programmed to monitor specific precursor ions and their corresponding fragment ions. This is achieved by selecting a precursor ion based on its mass-to-charge ratio (m/z) and then monitoring the specific fragment ions produced from its fragmentation. By selecting specific precursor and fragment ions, SRM enables the detection and quantification of specific peptides or proteins within a complex sample.
To know more about scientific visit:
brainly.com/question/33922284
#SPJ11
this reaction is an oxidation reduction reaction. mg 2hcl ---> mgcl2 h2 what substance is getting oxidized?
Answers
\(Mg + 2HCl\) -> \(MgCl_{2} + H_{2}\)
Redox reactions, also known as oxidation-reduction processes, involve the transfer of electrons from one species to another. The species that loses electrons is oxidized, whereas the species that gets electrons is reduced.Beside that, we can also identify redox reactions by assigning oxidation numbers to atoms in molecules and assuming that all bonds to the atoms are ionic. An increase in oxidation number during a reaction indicates oxidation, while a drop indicates reduction.In this case, Magnesium metal is being oxidized to magnesium cations (\(Mg^{2+}\)) and its oxidation number is increasing (from 0 on the reactants' side to +2 on the products' side).to know more about redox reactions, go to:
brainly.com/question/13293425
#SPJ4
The total internal energy of an ideal gas is 3770 J. If there are 3 moles of the gas at 1 atm, what is the temperature of the gas
Answers
The temperature of the gas is approximately 100.8 K. To find the temperature of an ideal gas, we'll use the formula for the internal energy of an ideal gas (U) and the ideal gas law. The given terms are the internal energy (U = 3770 J), the number of moles (n = 3 moles), and the pressure (P = 1 atm, which is approximately 101325 Pa).
First, we'll use the internal energy formula for an ideal gas: U = (3/2)*nRT, where R is the universal gas constant (8.314 J/mol·K). We're solving for the temperature (T), so we'll rearrange the formula to isolate T:
T = (2/3)*(U / nR)
Now, plug in the given values:
T = (2/3)*(3770 J / (3 moles × 8.314 J/mol·K))
T = (2/3)*(3770 J / 24.942 J/mol·K)
T = (2/3)*(151.213 K)
T = 100.809 K
Therefore, the temperature of the gas is approximately 100.8 K. This calculation assumes the gas behaves ideally and allows us to determine the temperature given the internal energy, number of moles, and pressure.
Learn more about internal energy here:
https://brainly.com/question/11742607
#SPJ11
match each statements with the state of matter it describes?
Answers
Answer:
Solid liquids , plasma gas
Explanation:
It retains its shape regardless of the shape of the container
How does the overall charge of oxygen-16
compare to the overall charge of oxygen-17 and oxygen-18? Use the
models of isotopes to help explain your answer

Answers
Answer:
there's a difference in neutrons in the neutron count
Explanation:
look at the diagram
The overall charge in both the isotopes is the same that is 0.
Explanation:
Isotopes are the atoms of the same element with the same number of protons and electrons but with different numbers of neutrons.In the isotope oxygen-17:
There are 8 protonsThere are 9 neutronsThere 8 electronsOverall charge on the oxygen-17:\(=+8+(-8)=0\)
In the isotope oxygen-18:
There are 8 protonsThere are 10 neutronsThere 8 electronsOverall charge on the oxygen-18:\(=+8+(-8)=0\)
The only difference in the given two isotopes is of number of neutrons
So from this, we concluded that the overall charge in both the isotopes is the same that is 0.
Learn more about the isotopes here:
brainly.com/question/24604793?referrer=searchResults
brainly.com/question/9280592?referrer=searchResults
What is the best statement about the data collected in Amir’s table?
A. Wave 3 resulted from destructive interference, and Wave 4 resulted from constructive interference.
B. Waves 3 and 4 resulted from constructive interference.
C. Waves 3 and 4 resulted from destructive interference.
D. Wave 3 resulted from constructive interference, and Wave 4 resulted from destructive interference.
Answers
Answer: The answer is D :)
Explanation:
Answer:
D
Explanation:
What is the molarity of a solution containing 5. 0 moles of solute in 469 mL of a soltution
Answers
molarity of a solution containing 5. 0 moles of solute in 469 mL of solution is 10.66 mol/L
We know that the Molarity of a given solution is -
Molarity = n/V-------------------(i)
where
n= number of moles
V = Volume of solution in liters
now as per the question-:
number of moles n = 5 moles
volume of solution V= 469 ml or 0.469 ml { convert volume given in ml to L }
putting the values in equation (i)
\(Molarity =\frac{5}{0.469}\)Molarity = 10.66 mol/L
Therefore Molarity of the given solution is 10.66 mol/L
For more information on molarity
https://brainly.com/question/30404105
what is the pattern relating the charge of the ion to the group to which it belongs?
Answers
Answer:
Explanation:
The column arrangement in the periodic table corresponds with the electron orbitals in which the electrons reside. There are labelled as s, p, d, and f, and have a series of energy shells that correspond to the rows from top (H: 1s) to bottom (Fr: 7s). Within each row are found one or more of the orbitals s, p, d, and f.
The first column has electrons in the s orbital for that energy level. This is the first appearance of any orbital for the indicated energy level. Thus the elements in the first column contain the first electron of a higher energy level for that entire row. This puts it in a position of relative instability as it is always seeking a lower enegy state. Opportunities abound in the world of elements for it to leave the parent atom, e.g. Na, and take up residence in a passing atom that would love an electron, say chlorine, Cl. Showing only the orbitals for the respective energy level, each element in column 1 has its last electron in the first available, higher-energy, orbital.
H: 1s^1
Li: 2s^1
Na: 3s^1
K: 4s^1
Rb: 5s^1
Cs: 6s^1
Fr: 7s^1
That is the life of the outer electron in all the first column elements. All of these elements are willing to lose [happy to lose] this single electron, thus giving the column elements a charge of +1 when the electron leaves.
A similar situation arises for the elements in column 17, the halogens (F, Cl, Br, I,At, Ts). Except all these elements have what is known as a nearly full valence shell of electrons. Cl, for example, is putting electrons into orbitals on the 3rd energy level. For Cl, this consists of the 3s and 3p orbitals. 2 electrons will fit into s orbitals, while 6 will fill the p orbitals.
F: 2s^2 2p^5
Cl: 3s^2 3p^5
Br: 4s^2 4p^5
I: 5s^2 5p^5
Column 17 elements are just 1 electron short of filling the p orbitals. The elements in column 18, the Noble Gases, all have full outer shells of electrons for that energy level. each has a p orbital filled with 6 electrons, the maximum allowed in the p orbitals. So for any one row, electrons are moved into orbitals that are on the outer energy level until all available orbitals are filled, whichj would be the Noble Gas column. Additional electrons would have to move to a higher energy shell after each row is completed.
[This leaves out discussion of the d and f orbitals which become available on row 4 for the columns noted as the Transition Elements.]
When a column 1 element loses an electron, it finds itself in a more stable, lower energy state. All the remaining orbitals are filled (in the energy levels preceding the one for the lone outer electron). This is a lower energy state for that element. Conversely, the halogens, when they gain an electon, are in a more stable state since all of their valence orbitals are now full. So the column 17 elements all exhibit simliar chemical properties and have a -1 valence charge.
Wordy, but true . . .
Derive the minimum value of r(cation)/r(anion) to ensure triangular crystal structure in ceramics. Show all steps
Answers
In order to derive the minimum value of r(cation)/r(anion) to ensure triangular crystal structure in ceramics, the following steps should be followed:
Step 1: Find the number of atoms in a unit cell of triangular structure
For the triangular structure, the number of atoms in a unit cell is 6. This is because each of the three corners of the triangle is occupied by an anion and at the center of the triangle there is a cation. Thus the number of atoms in a unit cell is given by 3(cations) + 3(anions) = 6 atoms
Step 2: Find the radius ratio of cation/anion for triangular structureIn a triangular structure, the cation is located at the center of the triangle, while the anions are at the corners of the triangle. The cation should touch the anions along the edges of the triangle. The minimum radius ratio is the radius ratio at which the cation touches the anions. This means that the distance between the center of the cation and the center of an anion is equal to the sum of their radii. This leads to the following relationship:
r_cation + r_anion = sqrt(3) * a/2 where a is the length of the edge of the triangle.
Solving for r_cation/r_anion, we get:r_cation/r_anion = sqrt(3)/2 - r_anion/a
Step 3: Find the minimum value of r_cation/r_anion for triangular structureIn order to ensure that the cation touches the anions, the minimum value of r_cation/r_anion is
1. This means that:r_cation/r_anion >= 1 or :r_cation >= r_anion
The minimum value of r_cation/r_anion is thus 1.
To know more about ceramics visit:
https://brainly.com/question/30509290
#SPJ11
Fill in the blank: The units Hertz (Hz) is used to express _________________________. options: wave period wavelength amplitude frequency
Answers
Answer:
Hertz is the rate at which current changes direction. SO frenquency
Explanation:
Greenhouse gases are gases that trap heat within the Earth's atmosphere. What would be the most likely result of an increase in the amount of greenhouse gases in the atmosphere? A. a reduction in global ocean circulation B. a decrease in global rainfall C. an increase in global temperature D. a change in global wind patterns
Answers
Answer:c.an increase in global temperature
Explanation:
Human activities add greenhouse gases to the atmosphere,trapping more heat than usual and contributing to global warming.It causes slight rises in average global temperatures which may lead to huge affects.
The most frequent effect is that glaciers and ice caps melt faster than usual.
why the population decrease as you go from bottom of the food chain to the top
Answers
Answer:
The population size decreases because the higher on the food chain one looks, the fewer the number of organisms that occupy that level.
Explanation:
:)
What type of reaction is Ch3ch3 + Cl2 -> CH3CH2Cl +Hcl
Answers
Answer:
displacement reaction..
Determine the dimensions of 7 . which is the viscosity of a liquid, by performing dimensional analysis of the following equation. F=2πrL
R
v
, eetc F is force (Kgm/s
2
) r is radius (m) L is length (m) v is speed (m/s) R is distance (m)
Answers
The dimensions of viscosity are kilograms per meter per second (kg/(m·s)).
To determine the dimensions of viscosity (symbolized as η), we can perform dimensional analysis on the given equation:
F = 2πrL / Rv
Breaking down the dimensions of each variable:
F: Force, [M][L][T]⁻²
r: Radius, [L]
L: Length, [L]
R: Distance, [L]
v: Speed, [L][T]⁻¹
Substituting the dimensions into the equation:
[M][L][T]⁻² = 2π[L][L][L] / [L][L][T]⁻¹ * η
Simplifying the equation:
[M][L][T]⁻² = 2π[L]⁴[T] * η
Equating the dimensions on both sides of the equation:
[M] = 2π[L]³[T]² * η
From this equation, we can see that the dimensions of viscosity (η) are:
[η] = [M][L]⁻¹[T]⁻¹
Therefore, the dimensions of viscosity are kilograms per meter per second (kg/(m·s)).
Learn more about viscosity from the given link
https://brainly.com/question/2568610
#SPJ11