Continental waters are classified into two categories Lentic and Lotic. Ecosystem has flowing waters; such as creeks, streams, rivers, springs, brooks and channels. A lentic Ecosystem has still waters such as ponds, marshes,ditches, reservoirs, and lakes. The ecosystem of Lentic and Lotic waters includes biotic (Living) interactions between plants, animals and micro- organisms, and abiotic (non living) physical and chemical interactions.
A) Fish
B) algae
C) Bacteria
D) water flow
Answers
Answer:
it is D
Explanation:
Related Questions
Au HSO3 nomenclatura
Answers
A 10-g sample of aluminum has a volume of 3.70 cm3. what is the density of aluminum?
Answers
Density = mass/volume
ρ = \(m/v\)
Density = 10/3.70
Density of aluminum 2.702 g/cm3.
What is aluminum ?A thin, silvery-white metal, aluminum is. It is soft and pliable. Aluminum is used in a wide variety of products, including cans, foil, kitchenware, window frames, beer kegs, and parts for airplanes.
In the Earth's crust, aluminum is the most prevalent metal and the third most abundant element. On Earth, it is typically found in substances and minerals such feldspar, beryl, cryolite, and turquoise. But mining minerals for aluminum is quite expensive.
Rust doesn't occur in aluminum. It's important to remember, though, that aluminum is a highly reactive metal in its purest form. Although pure aluminum technically dissolves when it comes into contact with water, its reactivity may also be its greatest strength.
To learn more about aluminum from the given link:
brainly.com/question/25869623
#SPJ4
What is one key physical difference between transition
metals and poor metals?
А.Atomic mass
B.Hardness
C.Reactivity
D.Charge
Answers
if 10g of a radioactive substance are present initially and 9 yes later only 5g remain, how much will be present after 14 yr
Answers
After 14 years, approximately 3.125 grams of the radioactive substance will remain.
The decay of a radioactive substance follows an exponential decay model. The decay rate is determined by the half-life of the substance. Let's assume the half-life of the substance is 1 year for simplicity.
In the given scenario, 9 years have passed, which is equivalent to 9 half-lives. Each half-life reduces the amount of the substance to half. Therefore, after 9 half-lives, only 10g * (1/2)^9 = 10g * 0.001953125 = 0.01953g (approximately 0.02g) remain.
Now, we need to calculate the remaining amount after a total of 14 years, which is equivalent to 14 half-lives. Using the same formula, we have: 10g * (1/2)^14 = 10g * 0.00006103515625 = 0.0006103515625g (approximately 0.00061g).converting grams to milligrams, we have approximately 3.125 mg remaining after 14 years.
Learn more about radioactive here:
https://brainly.com/question/1770619
#SPJ11
How to reduce the volume of hydrogen gas
Answers
At constant temperatures, the simplest approach to reduce the volume of a gas is to raise its pressure. So, at 700 bar, or 700 times normal atmospheric pressure, hydrogen has a density of 42 kg/m3, compared to 0.090 kg/m3 at normal pressure and temperature.
What can hydrogen gas eliminate?Hydrogen decreases metal oxides in the reactivity series below. That is, hydrogen can only decrease the oxides of metals that are less reactive than hydrogen itself.
High-Temperature Water Splitting: Chemical processes that split water to make hydrogen are fueled by high temperatures generated by solar concentrators or nuclear reactors.
The oxidation number of hydrogen gas is 0, but the oxidation state of hydrogen atoms in water is +1. As a result, the hydrogen atom has been oxidized. It acts as a reducing agent.
learn more about hydrogen gas refer
https://brainly.com/question/19813237
#SPJ1
5. Which is NOT true about mixtures?
A. Mixtures are physical combination of only one substance.
B. Mixtures can be homogeneous and heterogeneous.
C. Mixtures are physical combination of two or more substance.
D. Mixtures can be separated in different processes.
Answers
Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
A chemical substance is composed of one type of atom or molecule.
A mixture is composed of different types of atoms or molecules that are not chemically bonded.
A heterogeneous mixture is a mixture of two or more chemical substances where the various components can be visually distinguished.
A homogeneous mixture is a type of mixture in which the composition is uniform and every part of the solution has the same properties.
Various separation techniques exist in order to separate matter, including include distillation, filtration, evaporation and chromatography. Matter can be in the same phase or in two different phases for this separation to take place.
I need help NOWW PLEASE HELP ME




Answers
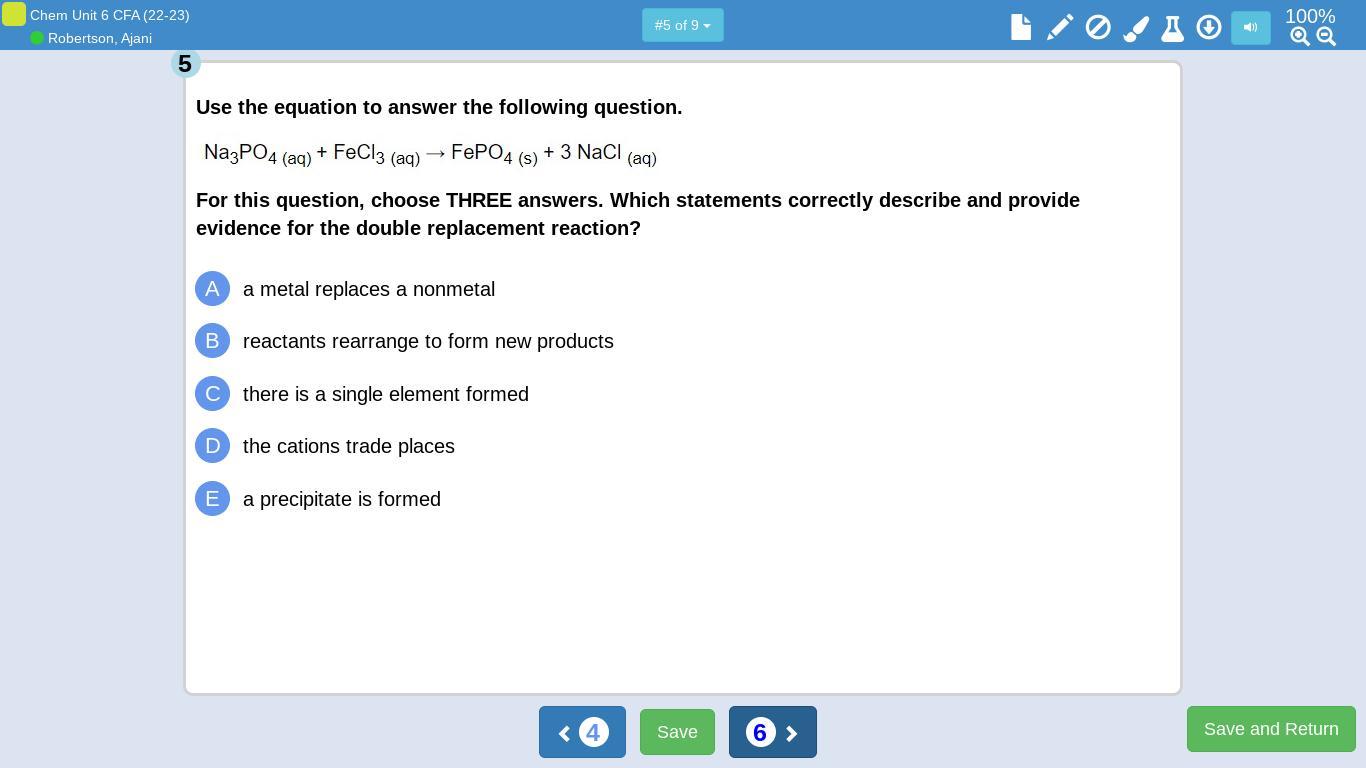
The reaction of Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. The correct formula of the compound with X having one valence electron and Z with 6 valence electrons is XZ₂. The given reaction in the third image is a single replacement reaction and the reaction in the fourth image is double displacement. Options A, B and E are correct for the reaction in fifth image.
What is displacement reaction?In displacement reactions , an atom or group is displaced by other atom or group from a reactant. In double displacement reactions, two group or atoms are displaced between two reactants.
In the reaction between Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. It is a single displacement reaction.
The element X with 1 valence electron will form a compound with Z having 6 valence electrons with the formula XZ₂. Z needs two electrons to attain octet. Thus two X provides one electron by each to Z.
The reaction of Na metal with HCl producing NaCl and hydrogen gas is a single displacement reaction. Whereas, the reaction between NaCl and MgF2 is double displacement because, both Cl and F atoms are displacing their position.
Sodium phosphate reacts with ferric chloride giving the ferric phosphate precipitate. Here the metals replaces nonmetals and the reaction involves formation of a precipitate. Thus, option A, B and E are correct.
To find more on displacement reaction, refer here:
https://brainly.com/question/3172917
#SPJ1
The molecular formula mass of this compound is 240 amu. What are the subscripts in the actual molecular formula?.
Answers
To calculate the subscripts in the actual molecular formula, we first have to find out the empirical formula of the given compound. Empirical formula mass is the sum of the atomic masses of all the atoms in a formula unit of the empirical formula of a compound.
To calculate the empirical formula mass, we add up the atomic masses of all the atoms present in a molecule of a compound. Using the molecular formula mass and empirical formula mass, we can calculate the actual molecular formula of the compound. The subscripts in the actual molecular formula are calculated by dividing the molecular formula mass of the compound by the empirical formula mass of the compound. Let's consider an example to understand how to calculate the subscripts in the actual molecular formula.
Example:
The empirical formula of a compound is CH2O, and the molecular formula mass of the compound is 180 amu. The empirical formula mass can be calculated using the formula:
Empirical formula mass = sum of atomic masses of atoms in the empirical formula
= (1 × atomic mass of C) + (2 × atomic mass of H) + (1 × atomic mass of O)
= (1 × 12.01 amu) + (2 × 1.01 amu) + (1 × 16.00 amu)
= 30.03 amu
Molecular formula mass = 180 amu
Now, we can calculate the ratio of the molecular formula mass to the empirical formula mass as:
180 amu ÷ 30.03 amu
= 6
The actual molecular formula will have six times the number of atoms as the empirical formula: C6H12O6. Therefore, the subscripts in the actual molecular formula of the compound are C6H12O6.
To know more about molecular formula visit:
brainly.com/question/28647690
#SPJ11
The subscripts of a molecular formula, where its mass is known, can only be determined when the elements in the compound are specified. The subscripts in this case are the ratios of these elements. Without these, one can only give hypothetical answers, like a carbon dioxide compound repeated five times which gives subscripts of 5 (C5O10).
Explanation:To deteremine the subscripts in the actual molecular formula, one first needs to know the formula of the compound itself. Without knowing the elements in the compound, we cannot ascertain the subscripts from the molecular formula mass. For example, if the compound is carbon dioxide (CO2), and knowing that the molecular mass of carbon (C) is approximately 12 amu and oxygen (O) is 16 amu, we know that two oxygens (16 * 2) with one carbon (12) has a total mass of 44 amu. Repeating this compound 5 times (as 240 is 5 times 44) would imply, in this hypothetical case, subscripts of 5, making the compound C5O10 . However, to get actual subscripts we need specifics about the compound's elements.
Learn more about Molecular Formulas here:https://brainly.com/question/35282824
#SPJ12
Students created the electromagnet you see here on the left. Mary's group had some bar magnets on the lab bench and brought one close to the electromagnet. Based on the illustration you see here, determine which statement is not correct. ( DO NOT GIVE ME A PATHETIC ANSWER)
Answers
Answer:
bro Ur in my school lol
Explanation:
Answer:
Explanation:
There will be no attractive or repulsive force between the two types of magnets. its A i took this quiz and got it correct. ^^
the_________ are new substances that are created following a chemical reaction
Answers
Answer:
products
Explanation:
when chemicals react new substances are created called PRODUCTS
Answer:
the _products_ are new substances that are created by following a chemical reaction.
Explanation:
^^
What is the name for a substance made of billions of oppositely charged ions
joined together?
Answers
Answer:
Ionic substance
Explanation:
An ionic substance is formed when oppositely charged ions link up to form an infinitely large lattice structure that can only be described in terms of unit cells.
Ionic substances may consist of billions of oppositely charged ions. Ionic substances are hard, have high melting and boiling points and do not conduct electricity in the solid state because the ions are not free in the solid state.
However, in solution or molten state, the substance conducts electricity since the ions which are the charge carriers are now mobile.
PLEASE HELP ASAP ILL RATE U 5 STARS!!

Answers
Using the periodic table, The Bohr - Rutherford diagram of each of the elements is before bonding and after bonding is attached below.NH\(_{3}\) ( ammonia ) is a covalent bond.
Ammonia is a covalent compound because in the formation of ammonia the bond between nitrogen and three hydrogen is formed by the sharing of electron.
Covalent bond is formed by sharing of electron between atoms. Ionic bond is formed when one atom loses electron and other atom gain the electron or by the transfer of electron from one atom to another.
Hence,In the case of ammonia a covalent bond is formed and the electronegativity difference between nitrogen and hydrogen is not big enough to make a covalent bond.
To learn more about Bohr-Rutherford diagrams here
https://brainly.com/question/14586294
#SPJ1


Which of the following elements has the largest second ionization energy (IE2)?
(a) Li
(b) B
(c) O
(d) F
(e) Na
Answers
Among the given elements, the element with the largest second ionization energy (IE2) is Oxygen (O).
The ionization energy refers to the energy required to remove an electron from an atom or ion. In general, the second ionization energy is higher than the first ionization energy because it involves removing an electron from a positively charged ion.
Oxygen (O) has an atomic number of 8, and its electronic configuration is 1s^2 2s^2 2p^4. When an oxygen atom loses one electron, it forms a positively charged ion, O^+, with the electronic configuration of 1s^2 2s^2 2p^3. To remove a second electron from O^+, it requires a significantly higher amount of energy compared to the other elements provided.
The trend for ionization energy generally increases across a period from left to right in the periodic table. This is because the effective nuclear charge increases, resulting in a stronger attraction between the nucleus and the valence electrons, making it harder to remove an electron. Oxygen (O) is located on the right side of the periodic table, and its second ionization energy is higher than that of lithium (Li), boron (B), fluorine (F), and sodium (Na).
Therefore, among the given elements, Oxygen (O) has the largest second ionization energy.
Know more about Second Ionization Energy here:
https://brainly.com/question/29799084
#SPJ11
what difference between satin nickel and brushed nickel
Answers
Satin nickel and brushed nickel are both finishes that give a smooth and matte appearance, but there is a slight difference in their texture. Satin nickel has a soft and almost velvety texture, while brushed nickel has visible lines or scratches that create a textured look.
\
The brushed nickel finish is achieved by using a wire brush to create those lines or scratches, while the satin nickel finish is achieved by applying a thin layer of lacquer or a similar substance to create a smooth, even finish. Overall, both finishes are great options for a modern and sophisticated look in your home, but it comes down to personal preference and the specific aesthetic you are trying to achieve.
The main difference between satin nickel and brushed nickel lies in the surface finish and appearance.
Satin nickel has a smooth, even, and slightly reflective surface finish that doesn't show visible brush strokes. It is created by applying a thin layer of nickel plating over a metal base, followed by a clear protective lacquer.
Brushed nickel, on the other hand, has a textured surface with visible brush strokes, giving it a more matte appearance. This finish is achieved by using a wire brush or abrasive pad to create a pattern of fine lines on the surface of the nickel plating.
Both finishes are popular choices for fixtures and hardware, as they provide a modern and elegant look while being resistant to tarnishing and wear.
learn more about nickel here
https://brainly.com/question/15616649
#SPJ11
What happens to the speed of the particles of the physics book?
Answers
Answer:
the physics book particales speed up and when you move the chemistry book
Answer:
The speed of the particles of the physics book increases when the chemistry book slides across the physics book.
Explanation:
i got it right when i did this
which chemical equation represents a redox reaction?

Answers
Answer:
B if i'm not mistaken.
Explanation:
a 600. ml beaker has an inner diameter of 73.0 mm. what is the vertical distance between the 100. ml marks on the side of the beaker? give your answer in cm.
Answers
There are 2.15 cm between each 100 mL mark. The formula; is used to determine a cylinder's molarity volume. V = πr^2h.
What is the straightforward meaning of molarity?The number of moles of a solute per liter of a solution is known as molarity. The molar concentration of a solution is another name for molarity.
What in chemistry is molality?The term "total moles of a solute contained in a kilogram of a solvent" is used to describe molality. Molal concentration is another name for molality. It gauges the amount of solute in a solution.
to know more about molarity visit:
https://brainly.com/question/8732513
#SPJ4
what mass (g) of agbr is formed when 35.5 ml of 0.184 m agno3 is treated with an excess of aqueous hydrobromic acid
Answers
Mass of AgBr is formed when 35.5 ml of 0.184 m agno3 is treated with an excess of aqueous hydrobromic acid is 1.23g.
What is Stoichiometric?We may calculate reactant and product amounts using balanced chemical equations thanks to the fundamental concept of stoichiometry. The balanced equation's ratios are used in this instance. Generally speaking, how a reaction will behave depends on the amount of substance present.
Equation formed:
AgNO₃(aq) + HBr(aq) ⇒ AgBr(s) + HNO₃(aq)
Calculate the amount of AgNO3 consumed throughout the reaction.
based on molarity (a 1000 ml solution of AgNO3 comprises 0.184 moles).
AgNO₃ moles in 35.5 ml solution = \(\frac{(0.184mol) . (35.5ml)}{1000ml}\)
∴ Moles of AgNO₃ = 0.006532 moles
Moles of AgBr formed by 0.006532 moles of AgNO₃
From balanced reaction eq.,
Moles ratio of AgNO₃ : AgBr = 1 : 1 (AgNO₃ = AgBr)
∴ Moles of AgBr = 0.006532 moles
Mass of 0.006532 moles of AgBr,
Molar mass of AgBr = 187.77g/ml
∵ Mass (AgBr) = 187.77 × 0.006532
Mass of AgBr = 1.23g
To know more about Stoichiometrics, visit:
https://brainly.com/question/15047541
#SPJ4
can you use benzoyl peroxide and salicylic acid together
Answers
Yes, benzoyl peroxide and salicylic acid can be used together. When used together, these two ingredients can be very effective in treating acne-prone skin.
Benzoyl peroxide is a powerful antibacterial agent that helps to reduce the number of bacteria on the skin, while salicylic acid is a keratolytic agent, meaning it helps to break down dead skin cells and unclog pores. Together, these two ingredients work to reduce the number of bacteria on the skin, unclog pores, and reduce inflammation associated with acne. It is important to note that these two ingredients should not be used in a single product. Instead, they should be applied separately at different times of the day. For example, benzoyl peroxide should be applied in the morning and salicylic acid should be applied at night. Additionally, it is important to be mindful of the concentration of each ingredient, as using too much of either can be irritating and cause dryness. Overall, using benzoyl peroxide and salicylic acid together can be a very effective way to reduce acne and clear up blemishes.
To learn more about benzoyl peroxide click here https://brainly.com/question/14711646
#SPJ4
a weak acid is 33 issociated at ph 5.0. what is the pka for this acid?
Answers
The pKa of a weak acid that is 33% dissociated at pH 5.0 is approximately 4.83.
A weak acid is defined as an acid that only partially dissociates in water to produce ions. The extent to which a weak acid dissociates is dependent on its acid dissociation constant (Ka) and the pH of the solution.
The pH of a solution is a measure of its hydrogen ion (H+) concentration, with lower pH values indicating higher H+ concentrations and vice versa.
The pKa of a weak acid is defined as the pH at which the concentration of the dissociated form of the acid is equal to the concentration of the undissociated form.
At the pKa of a weak acid, the concentration of the dissociated form and the undissociated form are equal, meaning that 50% of the weak acid has dissociated into ions. If we know the extent of dissociation at a particular pH, we can calculate the pKa using the equation:
pKa = -log10(Ka) = -log10([H+]/[HA])
where [H+] is the hydrogen ion concentration, [HA] is the concentration of the undissociated form of the acid, and Ka is the acid dissociation constant.
In this case, the weak acid is 33% dissociated at pH 5.0, meaning that [H+]/[HA] = 0.33. We can use this information to calculate the pKa:
pKa = -log10(0.33) = log10(1/0.33)
pKa = 0.52 + log10(3)
pKa = 0.52 + 0.48
pKa = 1.0
So, the pKa for the weak acid is approximately 4.83.
To know more about pKa here:
https://brainly.com/question/15074297#
#SPJ11
Now, drag a neutron to the center (marked x) representing the nucleus. what do you observe?
Answers
If we were to hypothetically drag a neutron to the center of the nucleus, marked as "x," it would result in an unstable configuration. This is because the nucleus of an atom is composed of protons and neutrons, which are held together by the strong nuclear force.
The strong nuclear force is responsible for binding the nucleus and counteracting the electrostatic repulsion between positively charged protons.
In a stable nucleus, the number of protons and neutrons is balanced, ensuring a relatively stable configuration. The ratio of protons to neutrons varies depending on the element, but generally, nuclei with a higher number of protons require a greater number of neutrons to maintain stability.
If we were to forcibly move a neutron to the center of the nucleus, it would disturb the delicate balance between the strong nuclear force and the electrostatic repulsion.
This disturbance would lead to an unstable configuration, and the nucleus would likely undergo some form of radioactive decay to achieve a more stable state.
The specific type of decay would depend on the characteristics of the nucleus and the excess of neutrons or protons. Possible types of decay include beta decay, in which a neutron is converted into a proton and emits a beta particle (electron or positron), or neutron emission, where the excess neutron is expelled from the nucleus.
It's important to note that dragging a neutron to the center of the nucleus is purely hypothetical and cannot be practically achieved.
The stability of the nucleus is maintained by the delicate balance of forces, and any disruption would result in the nucleus seeking a more stable configuration through radioactive decay.
Learn more about neutron in the link:
https://brainly.com/question/26952570
#SPJ11
slightly acidic ground water can dissolve limestone as it flows along joints and bedding planes to form caves. this reaction may then be reversed as water drips from the ceiling and splashes on the floor of an air-filled cave and minerals are precipitated to form features known as:
Answers
Slightly acidic ground water can dissolve limestone as it flows along joints and bedding planes to form caves. This reaction may then be reversed as water drips from the ceiling and splashes on the floor of an air-filled cave and minerals are precipitated to form features known as stalactite and stalagmite.
How do stalactite and stalagmite form?
Cave are formed underground chambers naturally. Most caves develop when slightly acidic ground water dissolves limestone along joints and bedding planes. It can take hundreds of thousand years to form.
Natural ground water is little acidic because of dissolved carbon dioxide (CO2) from the atmosphere or soil. Ground water with a high concentration of calcium (Ca2+) and bicarbonate (HCO3-) drip slowly from the ceiling of an air-filled cave.
Below is the chemical reaction of formation stalactite and stalagmite :
\(H_{2}O + CO_{2} + CaCO_{3} < - > Ca^{2+} + 2HCO_{3} ^{-}\)
A falling water drop, can precipitate calcite on both ceiling and the cave floor. As a water drop and hangs on the roof of the cave, some of the dissolved carbon dioxide lost into the cave's atmosphere. It causes the calcite to precipitate on the ceiling. Deposits of calcite on roof cave by dripping water are stalactite. In other word, stalactite is column of rock resembling an icicle which hang down from the ceilings of cave.
When the water drop falls to the cave floor, more CO2 loss and the calcite precipitate on the cave floor. Precipitation of calcite on floor cave named stalagmite. So, stalagmites are cone-shaped masses formed on cave floors, directly below stalactite.
Each subsequent drop add more calcite to the first deposits and make it continuously bigger. A stalactite grows downward while stalagmite grows upward.
Learn more about cave formation by clicking this link :
https://brainly.com/question/11862452?referrer=searchResults
#SPJ4

How does chemical weathering differ from physical weathering? A. It causes rocks to change color B. It increases the size of the rock C. It makes the rock stronger D. It makes the rock disappear
Answers
Answer:
A. It causes rocks to change color
Explanation:
Weathering can be defined as the gradual decomposition of rock. This means, rocks are broken into pieces or sediments through the process of weathering.
There are two types of weathering process:
1. Physical weathering also known as mechanical weathering. This is the breakdown of rocks into smaller pieces without changing the composition of the rock. Physical weathering is caused by the action of ice, wind, plant growth.
2. Chemical weathering: Here, the rocks changes not just in size of pieces but in its composition. This is when other minerals are applied on the rock in other to dissolve it.
What happens to iron in a bolt as the bolt rusts?
Answers
Answer:
write the following as fractions in their simplest form
select the valid ways to make an ammonia/ammonium buffer for use in the laboratory (select all that apply). select the valid ways to make an ammonia/ammonium buffer for use in the laboratory (select all that apply). mix equal volumes of 1 m nh3 and 1 m hcl mix equal volumes of 1 m nh3 and 0.01 m nh4 mix equal volumes of 1 m nh3 and 1 m nh4 mix some volume of 1 m nh3 and half as much 1 m hcl
Answers
We should mix equal volumes of 1 M NH₃ and 1 M HCl or to mix equal volumes of 1 M NH₃ and 1 M NH₄+ to make an ammonia/ammonium buffer.
To make an ammonia/ammonium buffer for use in the laboratory, the valid ways are:
1. Mix equal volumes of 1 M NH₃ and 1 M HCl.
- This combination will react to form the ammonium (NH₄+) and chloride (Cl-) ions, creating an ammonia/ammonium buffer.
2. Mix equal volumes of 1 M NH₃ and 1 M NH₄+.
- By combining ammonia (NH₃) and ammonium (NH₄+) in equal volumes, you can create an ammonia/ammonium buffer.
Therefore, the valid ways to make an ammonia/ammonium buffer are to mix equal volumes of 1 M NH₃ and 1 M HCl or to mix equal volumes of 1 M NH₃ and 1 M NH₄+.
To know more about ammonia, visit:
https://brainly.com/question/29519032#
#SPJ11
Define Electrical Energy.
Answers
Answer:
Energy made available by the flow of electric charge through a conductor.
Explanation:
what do all atoms of any single element have in common
Answers
Explanation:
They come in different kinds, called elements, but each atom shares certain characteristics in common. All atoms have a dense central core called the atomic nucleus. ... All atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is.
The common feature is that the atoms of all elements consist of electrons, protons, and neutrons.
What is an atom?An atom is a particle of matter that uniquely defines a chemical element.
An atom consists of a central nucleus that is usually surrounded by one or more electrons.
All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms.
For example, all helium atoms have two protons, and no other elements have atoms with two protons.
Learn more about an atom here:
https://brainly.com/question/1566330
#SPJ2
Nguyên tử của nguyên tố X có tổng số hạt 40. Tổng số hạt mang điện nhiều hơn số hạt không mang điện là 12 hạt.
Answers
It is found that 42.5 mL of 1.02 mol/L NaOH have been added to 50.0 mL of vinegar (ethanoic acid) when the phenolphthalein in the solution just turns pink. What is the concentration of the vinegar?
Answers
Answer:
0.867mol/L
Explanation:
Step 1:
The balanced equation for the reaction. This is given below:
CH3COOH + NaOH —> CH3COONa + H2O
From the balanced equation above,
The mole ratio of the acid (nA) = 1
The mole ratio of the base (nB) = 1
Step 2:
Data obtained from the question.
Volume of base, NaOH (Vb) = 42.5mL
Molarity of base, NaOH (Mb) = 1.02mol/L
Volume of acid, CH3COOH (Va) = 50mL
Molarity of acid, CH3COOH (Ma) =..?
Step 3:
Determination of the molarity of the acid solution.
This can be obtained as follow:
MaVa /MbVb = nA/nB
Ma x 50 / 1.02 x 42.5 = 1
Cross multiply
Ma x 50 = 1.02 x 42.5
Divide both side by 50
Ma = (1.02 x 42.5) /50
Ma = 0.867mol/L
Therefore, the concentration of the vinegar is 0.867mol/L.
Consider the reaction, h2 i2 => 2 hi , which is found to be half order in i2 and first order in h2. which step of the proposed mechanism must be slow in order to agree with this rate law?
Answers
3rd step of the proposed mechanism must be slow in order to agree with this rate law in the reaction, h2 i2 => 2 hi , which is found to be half order in i2 and first order in h2.
What is hydrogen?Having the atomic number 1 and the letter H, hydrogen is the first chemical element. The lightest substance is hydrogen. Hydrogen has the formula H2 and exists as a gas under normal conditions. It burns easily and is non-toxic, tasteless, colorless, odorless, and odorless.
With approximately 75% of all normal matter made up of hydrogen, it is the most prevalent chemical in the universe. The majority of hydrogen in plasma stars like the Sun is hydrogen. Water and organic compounds are two examples of molecules that contain the majority of the hydrogen that exists on Earth.
One proton, one electron, and no neutrons are present in each atom of the most prevalent isotope of hydrogen (symbol 1H).
Learn more about hydrogen
https://brainly.com/question/1426421
#SPJ4
Full question
Consider the reaction, H2 + I2 => 2 HI , which is found to be first order in I2 and first order in H2. Which step of the proposed mechanism must be slow in order to agree with this rate law?
I 2 => 2 I
I + H2 => H2I
H2I + I => 2 HI
1. 2
2. 3
3. 1
4. Either 2 or 3