Answers
Answer:
4 Co(s) + 3 O2(g) = 2 Co2O3(s)
Explanation:
The balanced equation for Co+O₂=Co₂O₃ is Co+2O₂=Co₂O₃
In the equation balancing of Co+O₂=Co₂O₃ ,some points needs to be taken
which involves ensuring that the number of elements on the left hand side is
the same as that on the right hand side.
In this scenario, the number of carbon is the same on both sides but the
difference is in the number of moles of oxygen in which 2 is added to the left
hand side to make it balanced.
Co+2O₂=Co₂O₃
Read more on https://brainly.com/question/21047043
Related Questions
I need help with this

Answers
As a result, the ideal gas law is applied, and the pressure of the gas in the container is 1.44 atm.
How does Charles Law compute pressure?The Kelvin temperature and hence the volume are going to be in direct proportion when the pressure on a sample of a dry gas is held constant, according to the definition of the Charles Law Formula. PV = k is the law's equation, and k might be a constant.
This issue can be resolved by applying the ideal gas law:
PV = nR
T = -52 °C + 273.15 = 221.15 K
n = 0.642 mol
V = 8.6 L
T = 221.15 K
\(R = 0.0821 L·atm/mol·K (gas constant for ideal gases)\)
PV = nRT
P = nRT/V
P = (0.642 mol)(0.0821 L·atm/mol·K)(221.15 K)/(8.6 L)
P = 1.44 atm
To know more about ideal gas visit:-
https://brainly.com/question/28257995
#SPJ1
An early arrangement of the then known elements was proposed by a British scientist John Newlands, which he called the Law of Octaves. Like other scientists at the time, Newlands arranged the elements in order of increasing atomic mass and noted that every eighth element had similar physical/chemical properties. In the modern Periodic Table, which of the following represents the last pair of elements for which Newlands' Law of Octaves would hold true?
Answers
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
J.J. Thomson's plum pudding model of the atom followed the discovery of
A)
negatively charged particles with a very small mass.
B)
positively charged particles with a very large mass.
0)
negative electrons that revolved around a positive nucleus.
D)
positively charged protons that were contained in a concentrated area.
Answers
J.J. Thomson's plum pudding model of the atom followed the discovery of negatively charged particles with a very small mass. Thus, the correct option for this question is A.
Who was J. J. Thomson?J.J. Thomson was a British physicist who discovered sub-atomic particles known as electrons within an atom. He also announced that atoms are made up of smaller components.
The plum pudding model is defined by Thomson in order to demonstrate that negatively charged sub-atomic particles known as electrons are surrounded by a volume of positively charged particles known as protons. It is one of the historical scientific model of the atom that governs all sorts of properties of sub-atomic particles.
Therefore, "negatively charged particles with a very small mass" is the discovery that follows J.J. Thomson's plum pudding model of the atom. Thus, the correct option for this question is A.
To learn more about the Plum pudding model, refer to the link:
https://brainly.com/question/4839193
#SPJ2
Which portion of a molecule of F2O has partial positive charge?
Question 3 options:
A)
The F atoms
B)
The central O atom
C)
The partial charge on each atom is zero
D)
The partial charge on each atom is negative
Answers
The partial charges on each fluorine atom are negative. Option B) The central O atom is the correct answer. Option B
The partial charges in a molecule are determined by the electronegativity values of the atoms involved. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In the case of \(F_2O\), fluorine (F) is more electronegative than oxygen.
Fluorine is the most electronegative element on the periodic table, meaning it has a high ability to attract electrons. Oxygen is also relatively electronegative but less so than fluorine. When fluorine atoms bond with oxygen, the shared electrons will be pulled more towards the fluorine atoms, creating a polar covalent bond.
In \(F_2O\), each fluorine atom will pull the shared electrons towards itself, resulting in a higher electron density around the fluorine atoms. This creates a region of partial negative charge around the fluorine atoms.
Conversely, the oxygen atom will have a region of lower electron density and, therefore, a partial positive charge. This is because the shared electrons spend more time around the fluorine atoms due to their higher electronegativity.
Option B
For more such question on partial charges visit:
https://brainly.com/question/29974793
#SPJ8
Which of these four elements is the most reactive
1: Na
2: Al
3: Rb
4: In
Answers
Answer:
1: Na
Explanation:
Out of the four elements, the most reactive element is sodium (Na).
Sodium is a highly reactive metal because it has only one valence electron in its outermost shell, which is relatively far from the positively charged nucleus. This makes it easy for sodium to lose its outermost electron and form a positively charged ion, which is why it readily reacts with other elements.
Aluminum (Al), rubidium (Rb), and indium (In) are also reactive metals, but they are less reactive than sodium.
Which of the following affects the potency of a drug?
The amount
Concentration
Number of exposures
Exposure method
Answers
Answer:
All of the listed factors can affect the potency of a drug.
Explanation:
All of the listed factors can affect the potency of a drug. Let's discuss each one:
The amount: The potency of a drug can be influenced by the dosage or amount administered. Generally, a higher amount of a drug can lead to a greater effect or potency. However, there may be optimal dosage ranges where the potency is maximized before plateauing or potentially causing adverse effects.
Concentration: The concentration of a drug refers to the amount of the drug present in a given volume or solution. A higher concentration of a drug can increase its potency since a greater quantity of the active substance is available to interact with the target receptors or sites.
Number of exposures: The number of times a person is exposed to a drug can also impact its potency. In some cases, repeated exposures can lead to an accumulation of the drug in the body, resulting in increased potency or stronger effects. However, this can also lead to tolerance, where the body becomes less responsive to the drug over time, requiring higher doses for the same effect.
Exposure method: The way a drug is administered or exposed to the body can affect its potency. Different routes of administration (e.g., oral, intravenous, inhalation, topical) can result in variations in the drug's absorption, distribution, and metabolism, which can influence its potency and onset of action.
It's important to note that potency is different from efficacy, which refers to the maximum therapeutic effect a drug can produce. Potency specifically relates to the amount of drug required to produce a particular effect.
An arctic weather balloon is filled with 34.1L of helium gas inside a prep shed. The temperature inside the shed is 5.°C. The balloon is then taken outside, where the temperature is â5.°C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1atm. Be sure your answer has the correct number of significant digits.
Answers
Answer:
When the balloon is taken outside if the outside temperature is lower than the temperature inside the shed, the volume of the helium gas will decrease according to Charles' law. However, if the temperatureoutside is higher than inside the shed, the volume of the helium gas in the weather balloons will increase.
Note: The question is not complete. Assumptionshas been made about the temperature.
Explanation:
According to Charles' law of gases, the volume of a given mass of gas is directly proportional to its temperature in Kelvin provided its pressure remains constant.
Mathematically, V1T1 = V2T2
Given that the volume of the helium gas inside the weather balloon is 34.1 L at a temperature of 5 °C;
When the balloon is taken outside if the outside temperature is lower than the temperature inside the shed, the volume of the helium gas will decrease according to Charles' law. However, if the temperatureoutside is higher than inside the shed, the volume of the helium gas in the weather balloons will increase.
Assuming, the temperature outside the shed was 6.5 °C:
V1 = 34.1 L, T1 = 5.°C or 5.0 × 10^-10 °C = 273.1500000005
V2 = ?, T2 = 6.5 °C 6.5 × 10^-10 °C = 273.15000000065
V2 = V1T1/T2
V2 = (34.1 × 273.1500000005) / 273.15000000065
V2 = 34.1 L
Due the very small increase in temperature, the volume of the balloon increases only very slightly that is increase that it is negligible.
How many moles of CO are needed to produce 209.7 moles Fe?
Answers
We must utilise the chemical formula for the reaction of iron and carbon monoxide to make iron oxide in order to respond to this question. It goes like this: CO + 2 Fe = Fe2O3. Therefore, 1 mole of CO is required for every 2 moles of Fe.
We must multiply the necessary 209.7 moles of Fe by 1/2 to determine the necessary moles of CO. We now have 104.85 moles of CO overall. We may utilise the stoichiometric coefficient for CO, which is 1, to verify our response. It is 1.
Accordingly, 1 mole of Fe2O3 is created for every mole of CO. As a result, 104.85 moles of Fe2O3 are created for every 104.85 moles of CO. because 2 moles If 104.85 moles of Fe2O3 are needed to make 1 mole of Fe2O3, then multiply that figure by two to find the amount of Fe that was created. This provides us the same quantity of Fe that we started with, or 209.7 moles, which supports our conclusion.
Learn more about moles at:
https://brainly.com/question/26416088
#SPJ1
A 12.2 mL sample of liquid was found to have a mass of 10.4 g. Calculate the density of this liquid ( in g/mL).
Answers
Answer:
d=m/
Explanation:
d is density, m is mass, v is volume
Given: m =10.4g, v=12.2mL
substituting in equation,
d=10.4/ 12.2
d=0.8524g/mL
To learn more about density:
The density of the liquid is 0.852 g/mL.
To calculate the density of the liquid, we need to use the formula:
Density = Mass / Volume
Given that the mass of the liquid is 10.4 g and the volume is 12.2 mL, we can substitute these values into the formula:
Density = 10.4 g / 12.2 mL
Simplifying this expression, we find:
Density = 0.852 g/mL
Density is a physical property of a substance and is defined as the amount of mass per unit volume. In this case, the density tells us that for every milliliter of the liquid, there is 0.852 grams of mass. The units of grams per milliliter (g/mL) indicate that the density is a ratio of mass to volume.It is important to note that the density of a substance can vary with temperature, so this value is only valid under the conditions at which the measurement was made. Additionally, the density can provide valuable information about the identity of a substance, as different substances have different densities.
for such more questions on density
https://brainly.com/question/26364788
#SPJ8
What was earth’s surface like? Landmasses? First land plants
Answers
Answer:
During the early Paleozoic Era, the Earth's surface was very different from what it is today. The continents were arranged differently, forming one large supercontinent called Pangea. This landmass was surrounded by a single large ocean called Panthalassa. The climate was much warmer and wetter than it is today, with no ice caps at the poles.
The first land plants, known as bryophytes, appeared during the early Silurian Period, around 430 million years ago. These plants were small and simple, lacking roots and vascular tissue. They grew in damp environments, such as along the edges of lakes and streams. They were important in the development of soils and in the colonization of land by other organisms, such as insects and other arthropods.
1. List the three measurements that are helpful when working with a gas.
Answers
Answer:
Three measurements that are helpful to know when working with a gas are volume, temperature, and pressure. container.
Hope this helps if its wrong correct me.
please Help me !! NO LINKS! fill this chemical reaction please
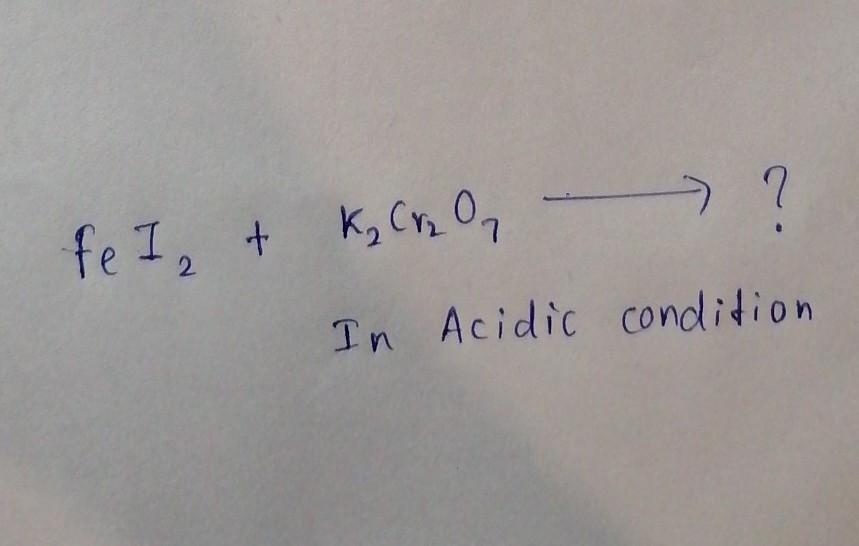
Answers
Answer:
see the above attachment.
Explanation:
hope this helps you.

Webb is testing samples of different elements. One sample is a dull, yellow solid that
breaks into powdery pieces when he hits it with a hammer. How should Webb classify
the yellow solid?
As a metal
As a nonmetal
As a gas
As a metalloid
Answers
Answer: it's nonmetal
Explanation: Sulfur has characteristics of nonmetals. It does not have luster, it cannot conduct electricity, and it is brittle. On the periodic table, sulfur is in group 16/VIA, in the third period. The group is called the oxygen group or the chalcogens.
Answer:
i dont know
Explanation:
A saturated solution of sucrose in 1000.0 g of boiling water is cooled to 20°C.
What mass of rock candy will be formed?
(Keep in mind that it’s solubility at 20° is 230.9)
Answers
No rock candy will be formed, as the solution is already at its maximum saturation point at 20°C.
We can calculate the maximum amount of sucrose that can be dissolved in 1000.0 g of water at this temperature as follows:
The maximum amount of sucrose that can be dissolved = \((230.9 g/100 mL) * (1000.0 mL/1000.0 g) = 2.309 g/g\)
This means that the maximum mass of sucrose that can be dissolved in 1000.0 g of water at 20°C is 2.309 g.
Mass of rock candy = Initial mass of sucrose - Mass of sucrose in solution at 20°C
Therefore, the mass of rock candy that will be formed is:
Mass of rock candy = Initial mass of sucrose - Mass of sucrose in solution at 20°C
\(= 2.309 g - 2.309 g/g * 1.000 kg \\= 0.0 g\)
To know more about saturation point, here
brainly.com/question/14118324
#SPJ1
Carbon monoxide and carbon dioxide have

Answers
Answer:
different chemical properties and the same physical properties
Explanation:
Both carbon monoxide and carbon dioxide are gases.
So physically, the two compounds have no fixed shape and volume and their molecules are far apart.
Therefore, they have similar physical properties.
Based on chemical properties, the compounds or molecules given are different.
They will combine chemically in diverse ways.
3. different chemical properties and the same physical properties
Physical properties:
Both carbon monoxide and carbon dioxide are gases. So physically, the two compounds have no fixed shape and volume and their molecules are far apart. Therefore, they have similar physical properties.Chemical properties:
The compounds or molecules given are different. They will combine chemically in diverse ways.Carbon Dioxide (CO₂) is a chemical compound consisting of one carbon atom and two oxygen atoms while Carbon Monoxide (CO) is a chemical compound which contains one carbon atom and one oxygen atom.CO is also a colorless and odorless gas while CO₂ is entirely human-made and is not naturally present in the atmosphere.Thus, both gases are chemically different.Therefore, option 3 is correct.
Learn more:
brainly.com/question/15821287
The Ksp for LaF3 is 2 x 10^-19. What is the solubility of LaF3 in water in moles per liter?
Answers
The solubility of\(LaF_3\) in water is 3.04 x 10^-6 mol/L.
The solubility of \(LaF_3\) in water can be determined using the Ksp expression:
\(Ksp = [La^{3+}][F^-]^3\)
Where \([La^{3+}]\)and \([F^-]\) are the molar concentrations of the \(La^{3+}\) and \(F^-\) ions in the solution.
Since each \(LaF_3\) formula unit dissociates into one \(La^{3+}\) ion and three \(F^-\) ions, the molar solubility of \(LaF_3\) can be represented as x. Thus, the molar concentrations of \(La^{3+}\) and \(F^-\) ions in the solution can be written as x and 3x, respectively.
Substituting these values into the Ksp expression gives:
Ksp = x*(3x)^3 = 27x^4
Now, we can solve for x:
x = (Ksp/27)^(1/4)
= (2 x 10^-19 / 27)^(1/4)
= 3.04 x 10^-6 mol/L
For more question on solubility click on
https://brainly.com/question/24057916
#SPJ11
number of SO42- ions in 20.4 g Cr2(SO4)3
Answers
✅
IamSugarBee
One mole of or 392 g of chromium sulphate gives 3 moles of sulphate ions. Thus 30 g of chromium gives 0.15 moles of sulphate ions or 9.39 ×10²² ions.
What are ions?Ions are charged substances formed by electron lose or gain by an atom. If an atom gain an electron it acquire one unit of negative charge and if it lose an electron it acquires a positive charge.
The ionization of chromium sulphate is written as follows:
\(Cr_{2}(SO_{4})_{3} \rightarrow 2Cr^{3+} + 3SO_{4}^{2-}\)
Hence, one moles of chromium sulphate forms 3 moles of sulphate ions. One mole of chromium sulphate is 392.18 g/mol. Thus number of moles 20.4 g of the compounds is 20.4 / 392.18 = 0.052 moles.
The number of moles of sulphate ions produced by 0.052 moles of chromium sulphate is calculated as follows:
0.052 × 3 = 0.15 moles.
One mole of sulphate ion contains 6.022 × 10 ²³ions. Thus number of ions in 0.15 moles is 0.15 ×6.022 × 10 ²³ = 9.39 ×10²² ions.
To find more about sulphate ions, refer the link below:
https://brainly.com/question/22698826
#SPJ2
7.00 of Compound x with molecular formula C3H4 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25c. The temperature of the water is observed to rise by 2.316c. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound x at 25c.
Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
Answers
Answer:
\(\Delta H_{f,C_3H_4}=276.8kJ/mol\)
Explanation:
Hello!
In this case, since the equation we use to model the heat exchange into the calorimeter and compute the heat of reaction is:
\(\Delta H_{rxn} =- m_wC_w\Delta T\)
We plug in the mass of water, temperature change and specific heat to obtain:
\(\Delta H_{rxn} =- (35000g)(4.184\frac{J}{g\°C} )(2.316\°C)\\\\\Delta H_{rxn}=-339.16kJ\)
Now, this enthalpy of reaction corresponds to the combustion of propyne:
\(C_3H_4+4O_2\rightarrow 3CO_2+2H_2O\)
Whose enthalpy change involves the enthalpies of formation of propyne, carbon dioxide and water, considering that of propyne is the target:
\(\Delta H_{rxn}=3\Delta H_{f,CO_2}+2\Delta H_{f,H_2O}-\Delta H_{f,C_3H_4}\)
However, the enthalpy of reaction should be expressed in kJ per moles of C3H4, so we divide by the appropriate moles in 7.00 g of this compound:
\(\Delta H_{rxn} =-339.16kJ*\frac{1}{7.00g}*\frac{40.06g}{1mol}=-1940.9kJ/mol\)
Now, we solve for the enthalpy of formation of C3H4 as shown below:
\(\Delta H_{f,C_3H_4}=3\Delta H_{f,CO_2}+2\Delta H_{f,H_2O}-\Delta H_{rxn}\)
So we plug in to obtain (enthalpies of formation of CO2 and H2O are found on NIST data base):
\(\Delta H_{f,C_3H_4}=3(-393.5kJ/mol)+2(-241.8kJ/mol)-(-1940.9kJ/mol)\\\\\Delta H_{f,C_3H_4}=276.8kJ/mol\)
Best regards!
A weather balloon is partially filled with helium to allow for
expansion at high altitudes. At STP, a weather balloon is
filled with enough helium to give a volume of 21.0 L. At an
altitude of 27.0 km and -44 °C, it has expanded to 2490 L.
The increase in volume causes it to burst and a small
parachute returns the instruments to the Earth.
What is the final pressure in mL of the helium inside the balloon when it bursts?
Answers
6.84mm Hg is the final pressure of the helium inside the balloon when it bursts.
Given that,
At STP, \(P_{1} = 1atm\)
\(T_{1}=273 K\)
\(V_{1}=25.0L\)
\(T_{2} =-44^{0} C=44+273=317K\)
\(V_{2} =2490L\)
By using the Combined gas law,
\(\frac{P_{1}V_{1} }{T_{1} } =\frac{P_{2}V_{2} }{T_{2} }\)
\(\frac{1atm*21.0L}{273K} =\frac{P_{2} *2490L}{317K}\)
\(P_{2} =\frac{1atm*21.0L*317K}{2490L*273K}\)
\(P_{2} =0.0097 atm\)
\(1atm=760 mm Hg\)
So, \(P_{2} =\frac{0.009atm* 760mm}{1atm} =6.84mm Hg\)
To learn more about Pressure visit: https://brainly.com/question/29073421
#SPJ1
The speed of light is 300,000 km/s. In scientific notation, this speed is written to one significant figure
as
3. × 10% km/s
C.
3.0 × 106 km/s
b. 3.0 × 105 km/s
d
3 × 105 km/s
Answers
Answer:
The answer should be 3.0x10^5
Explanation:
Please Brainlist.
How does adding a lone pair affect the position of existing atoms and lone pairs
Answers
Answer:
How does adding a lone pair affect the position of existing atoms and lone pairs? It decreases the angles between the atoms - the atoms are moving closer because they are being repelled further away from the lone pairs then they are from the other atoms
Explanation:
hope it helps!
What does the rate law tell you about a reaction?
A. How the concentration of the reactants affects the rate of a
reaction
B. How temperature affects the rate of a reaction
C. How the equilibrium constant is related to the rate of a reaction
D. How the rate of a reaction affects the total time of a reaction
Answers
Answer:
A. How the concentration of the reactants affects the rate of a reaction
Explanation:
Let's consider a generic reaction.
A + B ⇒ Products
The generic rate law is:
rate = k × [A]ᵃ × [B]ᵇ
where,
rate: rate of the reaction[A] and [B]: molar concentrations of the reactantsk: rate constanta and b: reaction ordersAs we can see, the rate law shows how the concentration of the reactants affects the rate of a reaction.
I can't copy and paste it because brainly responds with "Don't use such phrases here, not cool! It hurts our feelings =("

Answers
The true statements are;
The reaction is endothermic
Heat is a reactant
ΔHrxn is 89 kJ/mol
What is the enthalpy?Enthalpy is a thermodynamic property that represents the total energy of a system, including both its internal energy (the energy of its constituent particles) and the energy it has due to its temperature and pressure. It is commonly used in energy analysis and heat transfer calculations and is expressed in units of joules (J) or calories (cal).
We know that the enthalpy of the reaction can obtained from;
Enthalpy of the products - Enthalpy of the reactants
ΔH reaction = 2(-391.4) - 2(-435.9)
(-782.8) - (-871.8)
= 89 kJ/mol
Learn more about enthalpy: https://brainly.com/question/13996238
#SPJ1
If 1.00 g of KCl is completely dissolved in 24.5 g of water, what is the percent composition (by mass) of the solution that is formed?
Answers
Answer:
3.92%
Explanation:
The solution that is formed is of KCl in water. This means that the percent composition by mass is given by the formula:
Mass of KCl / Mass of Solution * 100%We now calculate the mass of solution:
Mass of Solution = Mass of KCl + Mass of Water = 1.00 g + 24.5 gMass of Solution = 25.5 gFinally we calculate the percent composition:
1.00 g / 25.5 g * 100% = 3.92%1) In the nuclear equation below, what does the letter X represent? Show your work.

Answers
What are the possible values of 1 and m for
n=4 ?
Answers
Answer:
If n = 4, then the possible values of 1 and m depend on the equation or expression being used. Without more information, it is impossible to determine what the possible values of 1 and m might be. Can you please provide more context or information about the problem you are trying to solve?
What is the probability of having major earthquakes or volcanoes occur in New York State?
Answers
Answer:
New York City is susceptible to at least a magnitude 5 earthquake once every 100 years.
Explanation:
The probability of having major earthquakes or volcanoes occur in New York State is at least, one magnitude 5 earthquake can occur in New York City once every 100 years.
What is earthquakes ?When two chunks of the ground abruptly slide past one another, an earthquake results. The fault or fault plane is the area where they slide.
Earthquakes often result from subsurface rock breaking unexpectedly and fast movement along a fault. The seismic waves that cause the ground to tremble are brought on by this quick release of energy.
The Circum-Pacific Belt, often known as the Ring of Fire, is a region in the Pacific Ocean marked by active volcanoes and frequent earthquakes. The Ring of Fire is where most of Earth's earthquakes and volcanoes occur.
Thus, at least, one magnitude 5 earthquake can occur in New York City once every 100 years.
To learn more about earthquakes, follow the link;
https://brainly.com/question/1176195
#SPJ2
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
Match the solution with the correct concentration.

Answers
Answer:
1. is Molar (with capital M)
2. is molal (m)
Explanation:
By definition, 1 Molar solutions have 1 mol of solute in 1 L of solution and 1 molal solutions have 1 mol of solute in 1 Kg of solvent