Arrhenius proposed that each reaction has an energy threshold that must be reached for the particles to react. The kinetic theory of gases proposes that the average kinetic energy of the particles is proportional to the absolute temperature. How do these concepts relate to the effect of temperature on rate?
Answers
The given concepts relate to the effect of temperature on rate as , when average kinetic energy of the molecules increases then rate will be also increases .
The reactant molecules will have a distribution of kinetic energy . The collision between molecules or the collision of molecules takes place . They also have a range of energies . So when the temperature increases, the fraction of molecules that have higher than the threshold energy increases and that have energy higher than the threshold also increases . So this also increases the number of collisions. So when the number of collisions increases the rate of the reaction increases this is how these two concepts are related to each other.
learn about average kinetic energy here
https://brainly.com/question/24134093
#SPJ4
Related Questions
The principle that allows the enthalpy of a reaction to be determined indirectly from several steps is called _____________
Answers
Answer:
The principle that allows the enthalpy of a reaction to be determined indirectly from several steps is called Hess's Law.
Explanation:
Hess's Law states that the enthalpy change of a chemical reaction is independent of the pathway between the initial and final states, as long as the initial and final conditions are the same.
This means that the overall enthalpy change for a reaction can be calculated by adding or subtracting the enthalpy changes for a series of simpler reactions that add up to the overall reaction.
TO KNOW MORE ON Hess's Law ,
https://brainly.com/question/14561923#
#spj11
i would Luke to ??

Answers
Answer:
1 answer
Explanation:
Which equation shows a physical change?
A-Pb(NO3)2 (aq) + 2KI(aq) → 2KNO3(aq) + PbI2(s)
B-2H2O(l) → 2H2(g) + O2(g)
C-H2O(g) → H2O(s)
D-HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Answers
Answer:
C
water has undergone a phase change and the composition of a substance was not changed
Examine the image. Which Indicator of a chemical change is shown?
Answers
An indicator of a chemical change which is shown in the image (see attachment) is: A.) formation of a gas.
What is a chemical change?A chemical change can be defined as a type of reaction that is typically characterized by a change in chemical composition and this leads to the formation of a new chemical substance.
This ultimately implies that, a chemical change would give rise to the chemical properties of matter by causing the transformation of one chemical substance into one or more different chemical substances.
In this context, we can infer and logically deduce that the formation of a gas is an indicator of a chemical change in the image (see attachment) shown below because the liquid turned to a gas when heat was applied.
Read more on chemical change here: https://brainly.com/question/19794032
#SPJ1
Complete Question:
Which indicator of a chemical change is shown?
A.) formation of a gas
B.) formation of a precipitate
C.) change in color
D.) change in energy

Hexane is a chain organic compound that has the formula C6H14. What type of organic compound is hexane? Alcohol Amine Ether Hydrocarbon
Answers
Answer: \(C_6H_{14}\) is a hydrocarbon.
Explanation:
Alcohols are the compounds which contain -OH as the functional group. Example: \(CH_3OH\)
Amine are the compounds which contain \(-NH_2\) as the functional group. Example: \(CH_3NH_2\)
Ether are the compounds which contain \(ROR\) as the functional group. Example: \(CH_3OCH_3\)
Hydrocarbons are defined as the compounds in which bonds are present between carbon and carbon atoms. Hydrocarbons with single bond between carbon and carbon atoms are called as alkanes.The general formula for alkanes is \(C_nH_{2n+2}\) Example: \(C_6H_{14}\) which is named as hexane.
Answer:
D) Hydrocarbon
Explanation:
Completed the test and got it right
Someone plz help me :(

Answers
Answer:
Descriptive investigations use careful observations and measurements to develop findings. Science journals, science logs, and field notebooks are some of the tools scientists use to gather information for descriptive investigations.
Explanation:
The __________ is the main control center for the autonomic nervous system. A. Forebrain B. Thalamus C. Hypothalamus D. Cerebrum Please select the best answer from the choices provided A B C D.
Answers
Answer:
its Hypothalamus
Explanation:
What is the name of Al2(CO3)3 ?
Answers
Answer: Aluminum Carbonate
Explanation:
Answer:
Aluminum carbonate.
Explanation:
It is an aluminum salt form of carbonic acid.
Rank the following atoms according to decreasing first ionization energy. (i.e. 1 = highest and 4 = lowest)
Options are Al, P, Mg, K?
Answers
The decreasing order of ionization energy will be Mg >Al >P>K.
Ionization energy sometimes referred to as ionization potential, would be the amount of energy it takes to eliminate an electron from a single, isolated atom or molecule.
On moving top to bottom in the periodic table, ionization energy will decrease rapidly.
Al =13 (group 3)
P =15 (group 5)
Mg =12 (group 2)
K =19 (group 1)
The decreasing order of ionization energy will be Mg >Al >P>K.
To know more about ionization energy.
https://brainly.com/question/18987798
#SPJ1
which of the following should be classified as a mixture?
Helium
Water
Iron
Air
Answers
Answer:
I'm pretty sure it's A)Helium
Explanation:
Answer:
AIR
Explanation:
Can someone help please?
Copper occurs naturally as a mix of two isotopes: Copper-63 with a mass of 62.930 amu and copper-65 with a mass of 64.928 amu. The percent abundance of copper-63 is 69.09%.
Calculate the atomic mass of copper.
Answers
Answer: 63.55
Explanation:
The attached chart shows the calculation. The orange line points to the % abundance of Cu-65, which is the difference of 100% less the % Abundance of Cu-65 (since there are only 2 isotopes).
The weighted average contribution of each istope is calculated (blue arrow) and then summed to find the atomic mass of copper.

Consider the following intermediate chemical equations.
Which overall chemical equation is obtained by combining these intermediate equations?
O CH(g)+20(g) →00:(g)+240(1)
CH(g)+20=(g) →CO:(g) + 2H2O(g)
240(g) 200
O CHI(g) +20%(g) →CO(g) +24:0(g)
O CHg)+20(g) →CO(g) + 4H+O(g) + 2H+O(10)
O CH4(g)+20(g) →CO:(g) + 6H0(g)
Save and Exit
Next
Answers
The combination of the equations would give us;
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
What do you get?We have the equations to be combined as;
CH(g) + 2O2(g) → CO2(g) + 2H2O(g)
CO(g) + H2O(g) → CO2(g) + H2(g)
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
This gives us;
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
This equation represents the complete combustion of methane, a process commonly used in energy production and heating systems. It illustrates how methane and oxygen react to form carbon dioxide and water vapor as the primary products.
Learn more about reaction equations:https://brainly.com/question/26996547
#SPJ1
for the galvanic cell pictured below, which statement is true? a. potassium ions from the salt bridge flow toward the fe electrode as the electrode is oxidized. b. potassium ions from the salt bridge flow toward the half-cell where fe3 is reduced. c. potassium ions from the salt bridge flow toward the cr electrode as the electrode is oxidized. d. potassium ions from the salt bridge flow toward the half-cell where cr3 is reduced.
Answers
The correct statement for the given galvanic cell is: b. potassium ions from the salt bridge flow toward the half-cell where Fe3+ is reduced.
1. In a galvanic cell, a spontaneous redox reaction occurs, where one half-cell undergoes oxidation (loses electrons) and the other half-cell undergoes reduction (gains electrons).
2. The salt bridge maintains electrical neutrality by allowing the flow of ions between the two half-cells.
3. The statement that potassium ions flow toward the half-cell where Fe3+ is reduced indicates that they are compensating for the increase in negative charge in that half-cell due to the reduction of Fe3+ to Fe2+ (Fe3+ + e- → Fe2+).
4. As electrons flow from the anode (oxidation half-cell) to the cathode (reduction half-cell), the salt bridge allows for the flow of cations (such as K+) to the reduction half-cell and anions (such as NO3-) to the oxidation half-cell, thus maintaining charge balance.
In conclusion, statement b is true because potassium ions from the salt bridge flow toward the half-cell where Fe3+ is reduced to maintain electrical neutrality during the redox reaction in the galvanic cell.
Learn more about electrical neutrality here:
brainly.com/question/13015042
#SPJ11
The formula for vitamin C is C6H6O6. Name the elements and the number of atoms of each element in one molecule of vitamin C.
Answers
Hydrogen - 6 atoms
Oxygen - 6 atoms
Question 7 (5 points)
(05.01 LC)
One mole of zinc has a mass of 65.4 grams. Approximately how many atoms of zinc are present in one mole of zinc?
Answers
Answer:
6.02 x 10²³ atoms
Explanation:
The number of moles present in 1 mole of zinc will be 6.02 x 10²³ atoms. This is because a mole of any substance contains the Avogadro's number of particles which is 6.02 x 10²³ atoms.
A mole is simple a convenient way of estimating the number particles within a substance.
So every 1 mole of a substance contains the same number atom in there.
For the give zinc, since it is 1 mole, it will contain 6.02 x 10²³ atoms
they've changed the answer, but 6.02 x 1023 is still right. the new answer is just 6 x 1023.(6.02 rounded to the nearest integer)
El ácido sulfúrico H2SO4 es uno de los compuestos que se utiliza para la producción de fertilizantes como el nitrosulfato amónico. Si disponemos de 8 mL de H2SO4 al 37 %P/P (d=1,26 g /mL), los cuales se disolvieron hasta alcanzar un volumen de solución de 400 mL, con una densidad de 1,08 g/mL. (La densidad del soluto es corresponde a 1,83 g/cm³)
Answers
Explanation:
12 hours ago
El ácido sulfúrico H2SO4 es uno de los compuestos que se utiliza para la producción de fertilizantes como el nitrosulfato amónico. Si disponemos de 8 mL de H2SO4 al 37 %P/P (d=1,26 g /mL), los cuales se disolvieron hasta alcanzar un volumen de solución de 400 mL, con una densidad de 1,08 g/mL. (La densidad del soluto es corresponde a 1,83 g/cm³)
how many moles are in 4.38 of AgNO2
Answers
Answer: 0.02 moles
Explanation:
The number of moles in 4.38 g of AgNO₂ is equal to 0.028 mol.
What is a mole?A mole can be described as a standard unit that can be utilized to calculate a given number of particles. The constituents counted are usually individually distinct but identical entities.
A mole of the substance can be used to calculate quantities of atoms, molecules, ions, or other particular particles. The quantity of matter can be described as measured by elementary entities.
The number of constituents present in one mole is equal to 6.023× 10 ²³ which is known as Avogadro’s constant.
Given, the mass of AgNO₂ = 4.38 g
The mass of one mole of AgNO₂ = 153.87 g
The number of moles of in 4.38 g of AgNO₂ = 4.38/153.87 = 0.028 mol
Therefore, the number of moles of AgNO₂ is equal to 0.028 mol.
Learn more about the mole, here:
brainly.com/question/26416088
#SPJ6
an electron moved from a lower energy lever to a higher energy lever, and then back down again. What most likely happened
a. first heat was absorbed from the lower level to the higher level, and then a photon was released when the electron moved back
down
b. first heat was released from the lower level to the higher level, and then a photon was released when the electron moved back
down
c. first a photon was released when the electron moved from the lower level to the higher level, and then a photon was absorbed
when the electron moved back down
d. first a photon was absorbed when the electron moved from the lower level to the higher level, and then a photon was released
when the electron moved back down
Answers
Answer:
d
Explanation:
How are large polymers formed? by repetitive combinations of simple subunits by the addition of halogens to a small molecule by combining a few different and larger molecules by combining many different and smaller molecules
Answers
Answer:
How are large polymers formed? by repetitive combinations of simple subunits by the addition of halogens to a small molecule by combining a few different and larger molecules by combining many different and smaller molecules
Explanation:
Which piece of evidence did Alfred Wegener's original theory of continental drift have access to?
seafloor spreading at mid-ocean ridges
reversing paleomagnetism in rocks on the ocean floor
a seafloor that was geologically active with earthquakes, volcanoes, and mountain chains
evidence of ancient tropical swamps in cold regions of North America
answer asap and there was no science so i had to pick wut was closest
Answers
Among the options provided, the piece of evidence that Alfred Wegener's original theory of continental drift had access to is:
Evidence of ancient tropical swamps in cold regions of North America
Alfred Wegener observed the presence of fossil evidence from ancient tropical plants, such as coal deposits and plant fossils, in regions that are currently cold, indicating that these regions were once located in different climatic zones.
This observation supported his idea of continental drift, suggesting that these continents were once connected and have since drifted apart.
Learn more about Drift here -: brainly.com/question/1638741
#SPJ11
hint: write a balanced chemical equation that illustrates the standard enthalpy of formation of n2o5. the standard molar internal energy of formation of n2o5(g) is 17.433 kj/mol at 298 k. what is the standard molar enthalpy of formation of n2o5(g) at the same temperature?
Answers
The standard molar enthalpy of the formation of N2O5(g) at 298 K is 19.913 kJ/mol.
To write a balanced chemical equation for the standard enthalpy of formation of N₂O₅, we start with the elements nitrogen and oxygen in their standard states:
N₂(g) + 5/2 O₂(g) → N₂O₅(g)
This equation shows that one mole of N₂ reacts with 2.5 moles of O₂ to form one mole of N₂O₅.
The standard enthalpy of formation, ΔHf°, is defined as the enthalpy change for the formation of one mole of a compound from its constituent elements in their standard states, all at 1 atm pressure and a specified temperature (usually 298 K). The enthalpy change can be calculated from the standard molar internal energy of formation, ΔUf°, using the equation:
ΔHf° = ΔUf° + RT
where R is the gas constant and T is the temperature in Kelvin.
Substituting the given values, we get:
ΔHf° = 17.433 kJ/mol + (8.314 J/mol*K)(298 K) / 1000 J/kJ
ΔHf° = 17.433 kJ/mol + 2.480 kJ/mol
ΔHf° = 19.913 kJ/mol
Therefore, the standard molar enthalpy of the formation of N₂O₅(g) at 298 K is 19.913 kJ/mol.
Learn more about standard enthalpy of formation at https://brainly.com/question/30431725
#SPJ11
A gas cylinder contains exactly 1 mole of oxygen gas (O2). How many molecules of oxygen are in the cylinder? 4. 01 × 1022 molecules 6. 02 × 1023 molecules 9. 03 × 1024 molecules 2. 89 × 1026 molecules.
Answers
The number of molecules on 1 mole of oxygen has been \(6.023\;\times\;10^2^3\) molecules. Thus, option B is correct.
The molecule has been the smallest unit of the compound. It has been the repeating unit to form the compound.
The molecule has been formed with the combination of the elements in the fixed proportion.
Number of molecules in a moleThe number of molecules in a mole of sample has been given by Avogadro's law. According to this, the number of molecules in a mole of sample has been equivalent to the Avogadro number.
The value of Avogadro number has been \(6.023\;\times\;10^2^3\).
Thus, the one mole of sample has been consisted of \(6.023\;\times\;10^2^3\) molecules.
The number of molecules on 1 mole of oxygen has been \(6.023\;\times\;10^2^3\) molecules. Thus, option B is correct.
Learn more about molecules, here:
https://brainly.com/question/19556990
Answer:
Answer in picture its B
Explanation:

Which substance has a melting point greater than room temperature
O A. argon
O B. oxygen
O
C. mercury
O D. aluminum

Answers
Aluminum is solid at room temperature and will, therefore, have a melting point that is above room temperature.
Melting pointThe room temperature is about 25 degrees Celsius.
Argon and oxygen are gases at room temperature.
Mercury is a liquid at room temperature.
Aluminum, on the other hand, is solid at room temperature. Hence, its melting point is definitely somewhere above room temperature.
More on melting points can be found here: https://brainly.com/question/25777663
which type of chemical reaction must be predicted using solubility rules?
Answers
Solubility rules are used to predict the outcome of precipitation reactions, which involve the formation of insoluble products.
Solubility rules are guidelines that help predict the solubility of different compounds in water. These rules are based on the general principles of ionic interactions and the solubility of common ionic compounds. When two soluble compounds are mixed together, a precipitation reaction may occur if the resulting compound formed is insoluble in water.
In precipitation reactions, the reactants are typically aqueous solutions of two different compounds. The solubility rules are used to determine if a precipitate will form by identifying the combination of ions that can form an insoluble compound. The rules state that certain combinations of ions will result in the formation of insoluble products, while others will remain soluble in water.
In conclusion, solubility rules are used to predict precipitation reactions, which involve the formation of insoluble products. These rules help determine whether certain combinations of ions will result in the formation of a precipitate or if the compounds will remain soluble in water. By understanding the solubility properties of different ions, scientists and chemists can predict the outcome of chemical reactions and better understand the behavior of substances in solution.
To learn more about Solubility refer:
https://brainly.com/question/23946616
#SPJ11
What is the name of Earth’s natural satellite?
Answers
Answer:
the moon is the earths natural satellite.
Explanation:
A natural satellite is in the most common usage, an astronomical body that orbits a planet, dwarf planet, or small Solar System body (or sometimes another natural satellite). Natural satellites are often colloquially referred to as moons, a derivation from the Moon of Earth.
Based on this, which of the reactions below
are double-replacement reactions?
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
2 AgNO3(aq) + Zn(8) + 2Ag(s) + Zn(NO3)2(aq)
Pb(NO3)2(aq) + 2KI(aq) + 2KNO3(aq) + Pb12(s)
BaCl2(aq) + Na2SO4 (aq) + BaSO4(s) + 2NaCl(aq)
Answers
Answer:
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
Explanation:
Chemical reactions are of different types namely; combination reaction, displacement reaction, double displacement reaction etc. Double displacement/replacement reaction is that reaction in which an exchange of ions occur between two reacting ionic compounds to form new products.
In this chemical reaction/equation;
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
2Cl- and SO42- are the ions exchanged in this reaction. Barium (Ba) displaces SO42-, while Sodium (Na) displaces 2Cl-, hence it is called DOUBLE DISPLACEMENT OR REPLACEMENT because the displacement involves two compounds/ions.
How to reduce the volume of hydrogen gas
Answers
At constant temperatures, the simplest approach to reduce the volume of a gas is to raise its pressure. So, at 700 bar, or 700 times normal atmospheric pressure, hydrogen has a density of 42 kg/m3, compared to 0.090 kg/m3 at normal pressure and temperature.
What can hydrogen gas eliminate?Hydrogen decreases metal oxides in the reactivity series below. That is, hydrogen can only decrease the oxides of metals that are less reactive than hydrogen itself.
High-Temperature Water Splitting: Chemical processes that split water to make hydrogen are fueled by high temperatures generated by solar concentrators or nuclear reactors.
The oxidation number of hydrogen gas is 0, but the oxidation state of hydrogen atoms in water is +1. As a result, the hydrogen atom has been oxidized. It acts as a reducing agent.
learn more about hydrogen gas refer
https://brainly.com/question/19813237
#SPJ1
HELP !!
Convert 84,520 g of Ne to atoms.
Answers
Answer:
2.53 x 10^24
Explanation:
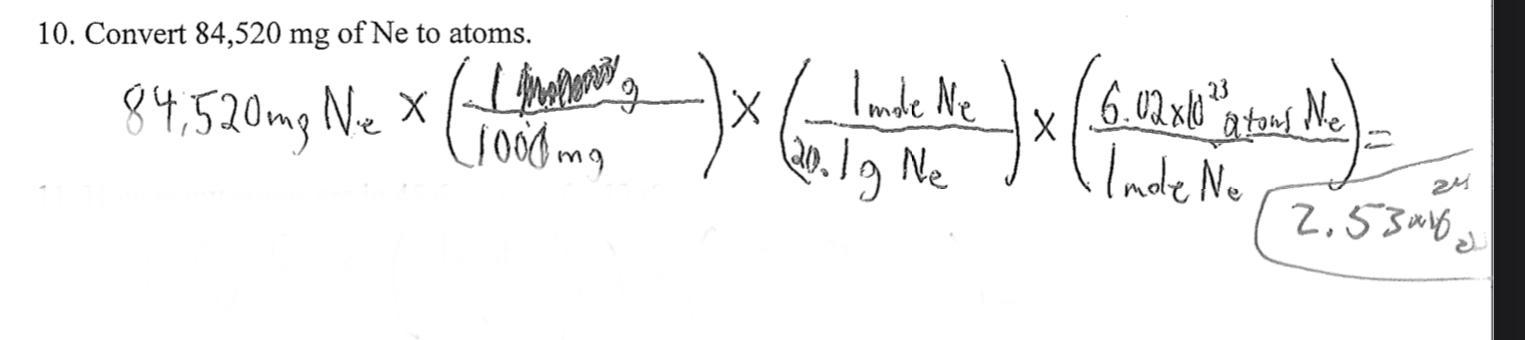
Fumaric acid, which occurs in many plants, contains, by mass, 41.4% carbon, 3.47% hydrogen, and 55.1% oxygen. The molecular mass of this compound is 116 amu. The molecular formula of this compound is
Question 15 options:
CHO
C3H3O
C3H3O3
C4H4O4
C6H6O6
Answers
Answer:
Explanation:
C = 41.4/12 = 3.43
H = 3.47/1 = 3.47
O = 55.1/16 =3.44
CHO is the skeletal formula (divide each by the lowest number above). The results are close enough to 1 to be 1.
(CHO)_x = 116
C + H + O = 29
(29) _ x = 116
x = 116/29
x = 4
So there area 4 carbons 4 hydrogens and 4 oxygens.
The correct formula is C4H4O4
What is the volume of a bar of soap that has a density of 2.5 g/cm3 and a mass of 100 g?
O4 cm3
O 0.4 cm3
O 400 cm3
O 40 cm3
Answers
The volume of a bar of soap that has a density of 2.5 g/cm3 and a mass of 100 g is 40 cm ³. Therefore, option D is correct.
What is density ?The mass of a substance per unit of volume is its density. A mechanically accepted measure is density. Density is most frequently represented by the symbol, however the Latin letter D may also be used.
The mass of a solid substance's unit volume. d = M/V, where d is density, M is mass, and V is volume, is the formula for density. Grams per cubic centimeter are a typical unit of measurement for density.
Density = m / V
volume = mass / density
= 100 / 2.5
= 40 cm³
Thus, option D is correct.
To learn more about the density, follow the link;
https://brainly.com/question/6107689
#SPJ1`