Answers
Answer: donates protons in a proton transfer reaction.
Explanation:
According to the Arrhenius concept, an acid is a substance that ionizes in the water to give hydrogen ion and a base is a substance that ionizes in the water to give hydroxide ion .
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
Phenolphthalein is an indicator which produces pink colour on adding into a base and turns colourless on adding to an acid.
Thus the only correct option is an acid donates protons in a proton transfer reaction.
Related Questions
What is the molecular formula of the product formed from the oxidation of 2-methyl-2,3-pentandiol with Jones reagent (CrO3, H , H2O)
Answers
Answer:
C6H12O2
Explanation:
The Jones reagent is a reagent in organic chemistry used to convert primary alcohols to carboxylic acids and secondary alcohols to ketones. Recall that tertiary alcohols can not be oxidized.
The compound 2-methyl-2,3-pentandiol contains one secondary and one tertiary alcohol. The secondary alcohol is oxidized to a ketone while the tertiary alcohol is not oxidized.
Hence the product of the oxidation using Jones reagent is 2-Hydroxy-2-methyl-3-pentanone with the molecular formula C6H12O2.
A science is considered interdisciplinary when
A. it's a hybrid between two fields of study.
B. a variety of experts from different scientific disciplines work together.
C. it considers different opinions when making decisions.
D. the sharing of data is required as a work is published.
Answers
Interdisciplinary science is a cooperative process that combines the knowledge and skills of qualified professionals from two or more fields.
How is interdisciplinary science important?Students that receive interdisciplinary instruction find it easier to understand scientific concepts and concerns that are presented in practical settings. Through the use of abilities and knowledge from any pertinent area, interdisciplinary teaching helps students deal with the problems.Merriam-definition Webster's is straightforward: "involving two or more academic, scientific, or creative domains." In essence, it means that you will be learning from two or more fields of study if you enrol in an academic programme that is defined (or partially defined) as interdisciplinary.Examples include bioinformatics, a field that combines computer science and molecular biology, and quantum information processing, a combination of quantum physics and computer science.To learn more about Interdisciplinary refer to:
https://brainly.com/question/28873816
#SPJ1
A phlebotomist has an order to collect STAT electrolytes and a CBC on a child. The draw is difficult and he is only able to collect a small amount of blood in a syringe. There is not enough blood to fill either of the collection tubes, so the phlebotomist places the blood in the appropriate microcollection containers instead. Which containers should be filled first? What additional important information is required on the specimen label and why?
Answers
Since there is only a small amount of blood available, the phlebotomist should prioritize filling the microcollection containers for the STAT electrolytes first.
Once the microcollection containers for the STAT electrolytes are filled, if there is any blood remaining, it can be used to fill the microcollection container for the CBC. The CBC typically requires a larger volume of blood compared to the electrolyte tests, which is why it is considered secondary in this situation.
On the specimen label, it is important to include additional information to ensure proper identification and processing of the specimen. The important information that should be included on the label includes:
Patient's name and unique identifier: This helps to ensure that the specimen is correctly matched to the patient and prevents any mix-ups or mislabeling.
Date and time of collection: This provides important information regarding the timeliness of the sample and allows for proper interpretation of the results.
Collection site or location: This information is helpful for tracking the origin of the specimen, especially if multiple collection sites are involved.
Phlebotomist's initials or identification: This helps in identifying the individual who collected the specimen, which can be useful for any follow-up or clarification needed during the testing process.
For more such question on phlebotomist visit:
https://brainly.com/question/31378339
#SPJ8
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
name the following : [Ni(NH3) 4(H2O)2(NO3)2
Answers
Answer: oxidation state of the transition metaliii
Explanation:
A 10.0 L container is filled with 0.40 mol H₂(g) 0.80 mol O2(g), and 0.80 mol SO2(g). If the total pressure
inside the flask is 816 torr, what is the partial pressure of the H₂(g)?
Answers
As a result, the container's partial pressure of Hydogen is 163.2 torr.
How can you determine the container's overall pressure?The partial pressures of the individual gases make up the total pressure of the gas combination. The total number of moles in the gas mixture, or ntot, is equal to the sum of all ni. Ptot = Pi = P1 + P2 + P3...
Calculating the total moles of gas in the container is the first step.
n(total) = n(H2) + n(O2) + n(SO2)
= 0.40 mol + 0.80 mol + 0.80 mol
= 2.0 mol
Next, we need to use the mole fraction of H2 to calculate its partial pressure:
X(H2) = n(H2) / n(total)
= 0.40 mol / 2.0 mol
= 0.20
The mole fraction of H2 is 0.20, so we can use this to find the partial pressure of H2:
P(H2) = X(H2) x P(total)
= 0.20 x 816 torr
= 163.2 torr.
To know more about pressure visit:-
https://brainly.com/question/23710615
#SPJ1
Part A A doctor orders 2.0 mg of morphine. The vial of morphine on hand is 30 mg per 6.0 mL How many milliliters of morphine should you administer to the patient? Express your answer to two significant figures and include the appropriate units.
Answers
(2.0 mg) / (x mL) = (30 mg) / (6.0 mL)
where x is the unknown number of milliliters we need to administer.
To solve for x, we can cross-multiply and simplify:
(2.0 mg) * (6.0 mL) = (30 mg) * (x mL)
12 = 30x
x = 12/30 = 0.4 mL
Therefore, we should administer 0.4 mL of morphine to the patient.
Which of the following names the quantity and correctly explains why that represents the astronauts weight
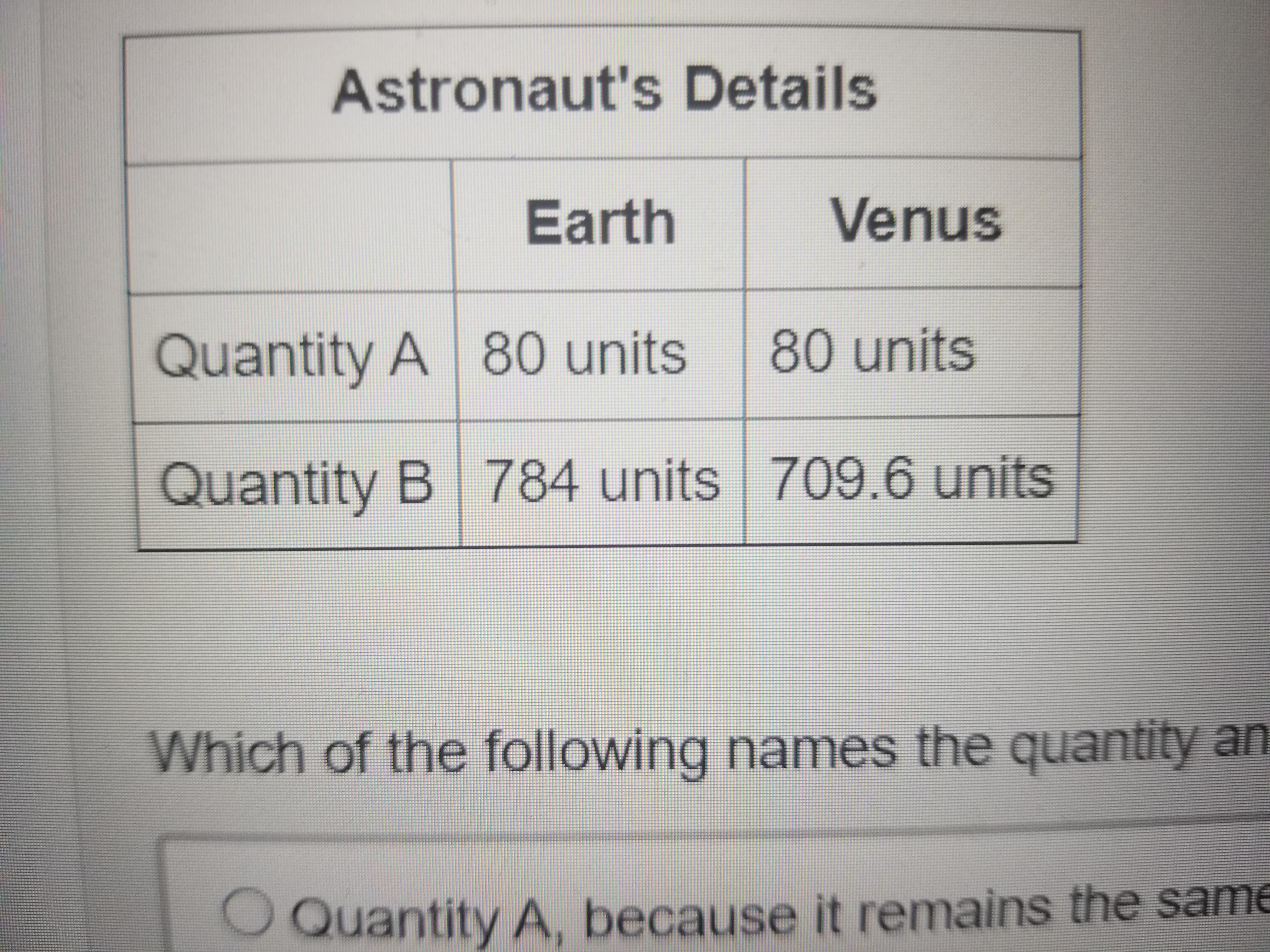
Answers
Answer:
b
Explanation:
How would you make the following solution? 500 g of solution of 0.150 m ZnCl2 from solid ZnCl2
Answers
The solution of 0.150 m ZnCl₂ can be prepared by the addition of 8.38 g of solid ZnCl₂ in 491.62 g of solvent.
What is molality?Molality can be measured by the number of moles of solute in a solution corresponding to 1 kg of solvent. The unit for molality in chemistry is mol/kg. A solution of concentration 1 mol.kg⁻¹ is also referred to as 1 molal.
The mathematical equation of the calculation of molality is given by:
Molality = Moles of solute/ Volume of solvent (in Kg)
Given, the mass of solution = 500 g
The molality of the zinc chloride solution = 0.125 m
The molar mass of the ZnCl₂ = 136.286 g/mol
Consider that the mass of solid ZnCl₂ is 'x'.
The mass of the solvent = 500 - x
\(Molality=\frac{x/136.286}{500-x}\times 1000\)
0.125 × 136.286× (500- x) = 1000x
1017.035 x= 8517.87
x = 8.38 g
The solvent = 500 - 8.38 = 491.62 g.
Therefore, the mass of the solute solid ZnCl₂ is 8.38 g should be added in 491.62 g of solvent to prepare 0.125 molal.
Learn more about molality, here:
https://brainly.com/question/26921570
#SPJ1
whats the strongest smell because the bodies in my basement is stinking really bad and i dont want people to find out can you recommend the best fabreeze or something like that
Answers
Answer:
ooooooooooppppppppppppppppppppppppp
Explanation:
Which of the following statements regarding energy is FALSE?
Energy is defined as the capacity to do work, or to put matter into motion
The products of exergonic reactions contain more potential energy than the reactants that form them
Increasing the temperature of atoms and molecules increases their kinetic energy
Exchange reactions allow chemical energy from one molecule to be transferred to another
Answers
Answer:
The correct answer is The products of exergonic reactions contain more potential energy than the reactants that form them.
Explanation:
It is considered false since the products in exothermic reactions have less energy than their reactants, since being exothermic they release energy in the form of heat to the environment that surrounds them.
An exothermic reaction gives energy to the environment that surrounds it at the end of the reaction, that is why the energy of the reactants will ALWAYS be greater than the final energy of the products, since as the reaction occurs, it loses energy and do not win.
The disorder and entropy in these reactions increases, complete combustion would be a clear example of exothermic reactions and their release of energy in the form of heat.
Exothermic reactions can be reversible or irreversible like all reactions.
I understand how a change in the size of the moon jellies' resource population can change the number of births in the moon jelly population.
Responses
Explain your answer choice.
Answers
A change in the size of the moon jellies' resource population can change the number of births in the moon jelly population because the big size of the resources can produce more births.
How do moon jellies reproduce?When there is more energy storage molecules present in the moon jellies, they can reproduce more, in more births. Fewer deaths would also lead to the jelly population increasing. The sea turtle population, and the moon jellies consumer population is also decreased.
There must be a change to the birth rate or the death rate in the moon jelly population. Within a population, organisms are born and dying continuously. If the number of births and deaths in a given time interval are equal, then the population size will remain stable.
So we can conclude that a large population of resources will lead to more births.
Learn more about jellies here: https://brainly.com/question/25630111
#SPJ1
name the organic molecules using nomenclature
please see picture
for the question.
thank you

Answers
a.) Pentane
b.) 2 - Methylpropane
c.) 3- Methylpentane
d.) Pent -2- ene
e.) 2 methylbut-2-ene
f. ) 3,3 dimethylpent -1-ene
g. ) 1 Bromo - 5- methylhexane
h. ) 3-Chloropentane
i. ) 1 - Iodoethane
Answer:
Explanation:
a.) Pentane
b.) 2 - Methylpropane
c.) 3- Methylpentane
d.) Pent -2- ene
e.) 2 methylbut-2-ene
f. ) 3,3 dimethylpent -1-ene
g. ) 1 Bromo - 5- methylhexane
h. ) 3-Chloropentane
i. ) 1 - Iodoethane
Separate samples of an unknown aqueous solution are mixed with solutions of HBr(aq) H_2SO_2(aq). and NaOH(aq). A solid precipitate forms in all three cases. Which of the following cations could the unknown solution contain?
Previous question
Answers
Double displacement response will shape a precipitate if the only of the goods is insoluble in water.
The solubility guidelines offer a listing of cations and anions that if blended the ensuing compound is both soluble or insoluble. The response equations between K+ and the 3 answers are given below.
HBr(aq)+K+(aq)→KBr(aq)+H+H2SO4(aq)+2K+(aq)→K2SO4(aq)+2H+NaOH(aq)+K+(aq)→KOH(aq)+Na+The response between Pb2+ and the 3 answers are given below.2HBr(aq)+Pb2+(aq)→PbBr2(s)+2H+H2SO4(aq)+Pb2+(aq)→PbSO4(s)+2H+2NaOH(aq)+Pb2+(aq)→Pb(OH)2(s)+2Na+The response between Ba2+ and the 3 answers are given below.2HBr(aq)+Ba2+(aq)→BaBr2(aq)+2H+H2SO4(aq)+Ba2+(aq)→BaSO4(s)+2H+2NaOH(aq)+Ba2+(aq)→Ba(OH)2(aq)+2Na+From the solubility guidelines, all the goods fashioned withinside the answer containing Pb2+ are insoluble, a precipitate will shape in all 3 cases.Read more about solutions;
https://brainly.com/question/25326161
#SPJ4
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
With the following data, construct a heating curve for the substance. Show relative differences and proper labels.normal melting point: 20 degrees celsius molar heat of fusion: 3KJ/molnormal boiling point: 126 degrees celsiusmolar heat of vaporization: 80KJ/mol
Answers
A heating curve gives us information on how much energy is required to change the state of matter of a substance and at what temperature this change occurs.
The x-axis represents the heat to be added and the y-axis the temperature. On the graph, when a phase change occurs, the temperature will remain constant, obtaining a straight line.
The melting point is the temperature at which a substance changes from a solid to a liquid state. The melting point will be linked to the molar heat of fusion.
The boiling point is the temperature at which there is a change of state from liquid to gas, the boiling point is linked to the molar heat of vaporization.
The heat curve for this case will be:

What is a technique for determining an unknown molaritry of acid by adding a base of know malarkey to a known volume of acid
Answers
Titration is a technique for determining unknown molarity of acid by adding a base of know malarkey to a known volume of acid
The process of calculating the quantity of a material A by adding measured increments of substance B, the titrant, with which it reacts until exact chemical equivalency is obtained " is the definition of titration. Titration, commonly referred to as titrimetry, is a method of chemical qualitative analysis used to determine the concentration of a certain analyte in a mixture.
The goal of titration is to identify the equivalence point, or the point at which chemically equivalent amounts of the reactants have been combined, which is a key analytical chemistry technique also known as volumetric analysis. The stoichiometry of the reactants determines how many reactants have been mixed at the equivalence point.
To learn more about titration please visit -
https://brainly.com/question/2728613
#SPJ1
pls someone help me when i get this done i am done with my work i will give brainlist to
Heat is often a by-product of an energy transfer, even when it is not intentional. Name one example where heat is an intentional product and one example where heat is not an intentional product of an energy transfer. Use details to support your answer.
Answers
Answer:
well i think coffe is an example cause it goes from hot to cold
Whats the answer 50 points and brainliest if its right

Answers
Answer:
D
Explanation:
A gas has an initial pressure of 3 atm at 6 L. If the pressure decreases to 1.5 atm, what will the new volume be?
Answers
Answer:
12 L
Explanation:
P1 V1 = P2 V 2
P1 V1 / P2 = V2
3 * 6 / 1.5 = 12 L
Sam built a rocket using a full set of blocks how would taking the rocket apart effect the total mass of the blocks
Answers
Answer:
The Answer is B
Explanation:
the total mass of the blocks would be the same apart as together.
As an electron in an atom moves from a higher energy state to a lower energy state, the atom
A
becomes a negative ion
В.
becomes a positive ion
С.
releases energy
D.
absorbs energy
Answers
Answer:B
Explanation:
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g. What is the density of the liquid to the appropriate number of significant figures
Answers
Answer:
0.420 g/ml
Explanation:
Question
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g. What is the density of the liquid to the appropriate number of figures
density = mass/volume
d = (192.1 -73.3)/140.0 = 58.8/140.0 =0.420 g/ml
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g.0.420 g/ml is the density of the liquid.
What is density ?The term density is defined as the mass of a substance per unit volume. density expressed in grams per cubic centimeter. The formula of density is as follows:
Density = mass/ volume
The density of something is a measure of how heavy it is in relation to its size. When an object is denser than water, it sinks; when an object is less dense than water, it floats. Density is a property of a substance that is independent of the amount of substance.
Given:
Volume = 140.0 cm³
Density = ?
mass = 73.3 g, 192.1 g.
density = mass/volume
density = (192.1 -73.3) / 140.0
= 58.8/140.0
=0.420 g/ml
Thus, The mass of the container and the liquid is 192.1 g.0.420 g/ml is the density of the liquid.
To learn more about the density, follow the link;
https://brainly.com/question/15164682
#SPJ2
3. Convert a measurement of 10 L to its equivalent in mL.
4. Convert a measurement of 1000 g to its equivalent in kg.
HELP!???!!!!
Answers
Answer:
3...... 1L = 1000ml
4. 1000g = 1 kg
Explanation:
mark me as brainliest ❤️
Convert 55 m3 to liters
Answers
Explanation:
hope it help you thanku byy.
.mark it brainlist

Which of the following factors contribure
to smog problems?
a. high numbers of automobiles
b. lots of sunlight
c. mountains surrounding urban areas
d. all of the above
Answers
Ground level ozone is created when sunlight reacts with certain chemicals that come from sources of burning fossil fuels, such as factories or car exhaust. When particles in the air combine with ozone, they create smog. Smog is a type of air pollution that looks like smoky fog and makes it difficult to see. Additionally, Cities located in basins surrounded by mountains may have smog problems because the smog is trapped in the valley and cannot be carried away by wind
sorting substances
below are some common substances. put in your experiences with these substances in the table below. we've filled out conductivity for you
Answers
Table salt does not melt on the stove, it dissolves in water and does not conduct electricity.
The nature of the substancesThere are several substances listed in the table Epsom salt is another one of these substances. It does not melt on a stove, it dissolves in water and also conducts electricity.
Finally, potassium chloride does not melt on the stove, it dissolves in water and it does conduct electricity. These are the experiences one can have with these common substances.
Learn more about conductivity here:
https://brainly.com/question/28869256
#SPJ1
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
When 91.96g of Na reacts with 32.o g of O2 how many grams of NaO2 are produced
Answers
Answer:
123.96 g Na₂O
Explanation:
4 Na + O₂ ⇒ 2 Na₂O
You first need to find the limiting reagent. Convert the reactants to moles and see which produces the least amount of product using the mole ratios in the chemical equation.
(91.96 g Na)/(22.99 g/mol Na) = 4 mol Na
(4 mol Na) × (2 mol Na₂O/4 mol Na) = 2 mol Na₂O
(32.0 g O₂)/(32.0 g/mol) = 1 mol O₂
(1 mol O₂) × (2 mol Na₂O/1 mol O₂) = 2 mol Na₂O
Since they both produce the same amount of product, you don't need to pick a limiting reagent. Now, convert moles of Na₂O to grams.
(2 mol Na₂O) × (61.98 g/mol Na₂O) = 123.96 g Na₂O
What is the name of the compound H3N?
Answers
Some research I did on Ammonia:
What is Ammonia? Ammonia, NH3, is a chemical compound composed of one nitrogen atom and three hydrogen atoms. Ammonia is a colorless gas that is lighter than air, and can be easily liquefied. In humans, ammonia from deaminated amino acids is quickly converted to urea, a less toxic form. The chemical formula for ammonia is NH3.
The compound H₃N is named "ammonia."
Ammonia is a colorless gas with a distinct pungent odor. It is composed of one nitrogen atom (N) and three hydrogen atoms (H), which form a covalent bond. It is commonly referred to by this name in both scientific and everyday usage.
Ammonia is composed of one nitrogen (N) atom and three hydrogen (H) atoms. It is a covalently bonded compound, meaning the nitrogen and hydrogen atoms share electrons to form the chemical bonds.
In its pure form, ammonia is a colorless gas with a distinct pungent odor. It is highly soluble in water and can easily dissolve in many other solvents.
To learn more about the ammonia, follow the link:
https://brainly.com/question/12276882
#SPJ6