a chemical formula contains information about the relative numbers of each type of atom in a compound. complete the following stoichiometric relationships.
1 mol NH3 contains ... mol N and .... mol H.
Answers
In 1 mole of NH₃:
It contains 1 mole of nitrogen (N).It contains 3 moles of hydrogen (H).
So, 1 mol NH₃ contains 1 mol N and 3 mol H.
In 1 mole of NH₃, we have one mole of the nitrogen atom (N) and three moles of the hydrogen atom (H).This means that for every 1 mole of ammonia (NH₃) that we have, we will obtain 1 mole of nitrogen (N) atoms and 3 moles of hydrogen (H) atoms. The ratio of nitrogen to ammonia is 1:1, while the ratio of hydrogen to ammonia is 3:1.
To further illustrate this, Consider a numerical example. If we have 2 moles of NH₃, then we can determine the corresponding number of moles for nitrogen (N) and hydrogen (H).For nitrogen:
2 moles NH₃ * (1 mole N / 1 mole NH3) = 2 moles N. For hydrogen:
2 moles NH₃ * (3 moles H / 1 mole NH3) = 6 moles H. So, in 2 moles of NH₃, we would have 2 moles of nitrogen (N) and 6 moles of hydrogen (H).This stoichiometric relationship is crucial in chemical calculations, as it allows us to determine the quantities of different elements present in a compound based on its chemical formula.
Learn more about stoichiometric relationships here, https://brainly.com/question/14935523
#SPJ11
Related Questions
In a chemical equation, the symbol that means "dissolved in water" is _____. (s) (l) (aq) (g)
Answers
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
pls-help..................



Answers
Answer:
Ok, in number 1 you have to write down the characteristics of light listed there in the Venn diagram, and place them in each column.
In number 2, you have to color in the boxes for with the corresponding color. For example, in box R, you would color red, and so on. Then, you have to color the wave of light following your chart on top of it.
In number 3 you have to color the apple red, and color the sun yellow and draw a yellow ray from the sun hitting the apple and then changing into red and bouncing into the eye.
Explanation:
I hope this helps.
Please give Brainly.
Ok I gave you the answer to question 2 and 3, the answer to question 1 is:
cannot be seen is ultraviolet light, can be seen is visible light, higher energy is ultraviolet light, lower energy is visible light, from of electromagnetic energy is both, emitted from the sun is ultraviolet light. :)
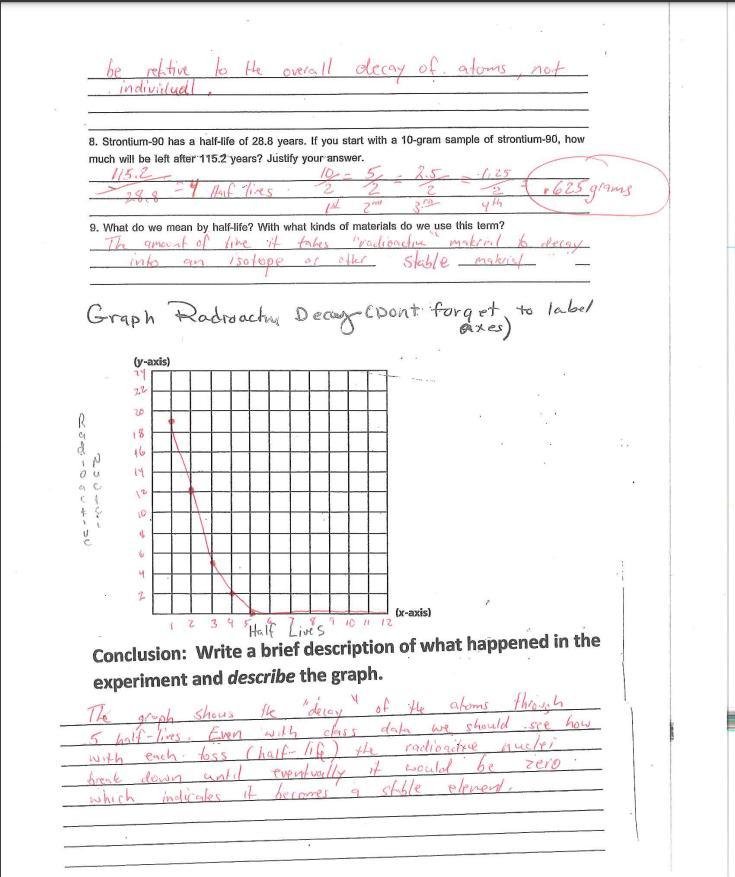
6. Is there any way to predict when a specific piece of candy will land marked
side up or "decayed?" If you could follow the fate of an individual atom in a
sample of radioactive material, could you predict when it would decay?
Explain.
Answers
Answer:
No, each piece of candy has a 50% chance of decaying.No, just like the candy, each atom has a 50% chance of decaying each half-life.Explanation:

the reaction between copper and silver nitrate produces beautiful silver flakes and a bright blue copper (ii) nitrate solution according to the following reaction: cu 2agno3 2ag cu(no3)2 how many grams of cu will be required if a jeweler wants to use this reaction to produce 25.0 grams of ag for a new piece of jewelry?
Answers
= 7.36 g of Cu
Moreover, Copper is a chemical element with the symbol Cu and atomic number 29. It is a soft, malleable and ductile metal with very high thermal and electrical conductivity. The freshly exposed surface of pure copper has a pinkish-orange color.
Copper is one of the few elements found in nature in essentially pure form. These samples of copper metal are on display at the Smithsonian Museum of Natural History.
you can learn more through link below:
https://brainly.com/question/15097782
#SPJ4
Which type of compound can be made in one step from a secondary alcohol?
A. an aldehyde
B. an alkane
C. a carboxylic acid
D. a ketone
Answers
The type of compound that can be made in one step from a secondary alcohol is a ketone. This is because the process of oxidizing a secondary alcohol results in the formation of a ketone.
Oxidation is a chemical reaction that involves the loss of electrons, and in the case of secondary alcohols, the loss of two electrons from the hydroxyl group (OH) results in the formation of a carbonyl group (C=O) that characterizes ketones.The reaction can be carried out by using various oxidizing agents such as chromic acid, potassium permanganate, or sodium dichromate. The choice of the oxidizing agent depends on the specific secondary alcohol being used and the desired end product. However, it is important to note that the reaction conditions need to be carefully controlled to avoid over-oxidation, which can lead to the formation of a carboxylic acid.In conclusion, a ketone can be made in one step from a secondary alcohol through oxidation. This process is commonly used in organic synthesis to create various compounds that have different applications in industries such as pharmaceuticals, perfumes, and flavors.Hi! The compound that can be made in one step from a secondary alcohol is D. a ketone. When a secondary alcohol undergoes oxidation, it is converted into a ketone.
Learn more about alcohol here
https://brainly.com/question/947751
#SPJ11
PLS HELP ME WITH QUESTION 5 I NEED HELP ASAP

Answers
Answer:
30.5%
Explanation:
Ionic compounds tend to be ...
A. Hard
B. Soft
C. Bendable
Answers
Answer:
A
Explanation:
Answer:
A. Hard
Explanation: got it correct on Edg
IM SO CLOSE TO FINISHING PLEASE HELP what type of reaction is KOH + H₃PO₄ --> K₃PO₄ + H₂O
Answers
Answer:
double displacement reaction also neutralization reaction cause of acid and base
Answer:
Double Replacement Reaction
Explanation:
KOH + H₃PO₄ --> K₃PO₄ + H₂O
AB + CD --> AD + BC
K goes with PO4 and H goes with OH
which statements regarding complex ions are true? select all that apply. select all that apply: the metal in a complex ion acts as a lewis acid, and the ligand acts as a lewis base. the ligands must be negatively charged. only transition metals can form complex ions. coordinate covalent bonds are the primary interaction between the lewis acid and lewis base in complex ions.
Answers
The statements regarding complex ions that are true c)only transition metals can form complex ions.
A complex ion is an ion that consists of one or greater ligands which are connected to a primary metallic cation via a dative covalent bond. A ligand is a species which can shape a dative covalent bond with a transition metallic the use of its lone pair of electrons. A complicated ion bureaucracy from a metallic ion and a ligand due to a Lewis acid–base interaction. The definitely charged metallic ion acts as a Lewis acid, and the ligand, with one or greater lone pairs of electrons, acts as a Lewis base.
To learn more about complex ion check the link below-
https://brainly.com/question/24262383
#SPJ4
Answer:
The metal in a complex ion acts as a Lewis acid, and the ligand acts as a Lewis base.
Coordinate covalent bonds are the primary interaction between the Lewis acid and Lewis base in complex ions
Explanation:
The metal (cation) in a complex ion accepts a pair of electrons from the ligands. This means the metal acts as a Lewis acid, and the ligands act as Lewis bases. The Lewis base donates a pair of electrons to the Lewis acid, forming a coordinate covalent bond between ligand and metal.Ligands are Lewis bases (electron pair donors). They can be negatively charged (such as OH−) or neutral molecules (such as H2O).Metals act like Lewis acids in complex ions. The metals need not be transition metals. For example, aluminum (Al3+) is a main group element that can act like a Lewis acid to form complex ions.
5. Hydrogen is placed in group 1 in the modern periodic table.
Answers
Answer:
how is this a question this is more of a statement but is this is a question the answer would be yes that is correct.
Explanation:
because in the periodic table that is where hydrogen stands.
please help i’m struggling

Answers
Answer:
Micro, milli, deci, deca, mega
Explanation:
the suffix each denote the amount in comparison to a liter
After the removal of carbon, the oxygen in co2 ends up.
a. true
b. false
Answers
The given statement, After the removal of carbon, the oxygen in CO₂ ends up is False.
Carbon dioxide (CO₂) is a compound made up of two elements, carbon and oxygen, in a ratio of one carbon atom for every two oxygen atoms. When carbon dioxide is removed, the oxygen atoms do not remain isolated - instead, they bond with other oxygen atoms from the surrounding environment, forming oxygen gas (O₂).
Oxygen gas is highly reactive and forms strong bonds with other oxygen atoms to form molecules of the natural gas O₂. The result is that the oxygen that was part of the carbon dioxide is no longer present - it has become part of the newly formed oxygen gas molecules.
Oxygen gas is present in the atmosphere and it is highly reactive and mobile, meaning that it can quickly move and form bonds with other elements. When carbon dioxide is removed, the oxygen atoms that were part of the molecule become part of oxygen gas instead, creating molecules of the natural gas O₂.
know more about environment here
https://brainly.com/question/5511643#
#SPJ11
The average speeds of gas molecules in cylinders A, B, C, and D are 0. 001 m/s, 0. 05 m/s, 0. 1 m/s, and 0. 0005 m/s respectively. Which cylinder contains gas that is closest to absolute zero? A B C D.
Answers
The cylinder that contains gas closest to absolute zero temperature is cylinder D.
The correct option is cylinder D, 0. 0005 m/s.
What is gas?Gas is one of the four states of matter. Gas is made up of individual atoms. The molecules of gas are very far from each other.
There are many gases present in the atmosphere.
The speed of gas depends on the temperature of the cylinder. The lower the temperature, the slower the speed of the molecule of gas.
Thus, cylinder D (0. 0005 m/s) has the slowest speed, which is related to low temperature absolute zero.
Learn more about gas, here:
https://brainly.com/question/13123721
Why is it advised to add concentrated acid to water and not water to the acid?
Answers
Acid first forms a highly concentrated solution when water is added to it, and this solution may vigorously boil, spitting concentrated acid. Acid and water combine to form an incredibly dilute solution that cannot be vaporized and spattered because of the insufficient heat output.
An exothermic reaction occurs when water is introduced to a concentrated acid; the heat that is produced may cause the liquid to splash out and cause burns. In contrast, the mixture won't splash out when acid is gradually added to water while stirring continuously since less heat is produced. Concentrated acid is splashed out of the container when water is added to the solution, which causes the solution to violently boil. Here, hydration results in an exothermic process that releases a significant quantity of energy. As a result, concentrated acid is introduced to water instead of the other way around.
Learn more about exothermic reaction here:
https://brainly.com/question/10373907
#SPJ4
The solubility for KCl is 60 g at 40 C. If a solution of KCl contains 79.5 g, is the solution saturated, unsaturated, or supersaturated
Answers
Answer:
saturated
Explanation:
Barium fluoride (BaF2) has a Ksp = 2. 5 × 10–5 (mol/L)3.
i. Write the dissolution reaction of BaF2, including all states. (0. 5 points)
ii. Write the expression for Ksp for BaF2. (0. 5 points)
iii. Write the Ksp expression as an algebraic equation, using the variable x to represent concentrations. (1 point)
iv. Find the solubility of BaF2 by solving the equation you wrote in part iii. (0. 5 points)
Answers
This problem is asking for the dissolution reaction of barium fluoride, both the equilibrium and Ksp expressions in terms of concentrations and x and its molar solubility in water. Thus, answers shown below:
\(BaF_2(s)\rightleftharpoons Ba^{2+}(aq)+2F^-(aq)\)\(Ksp=[Ba^{2+}][F^-]^2\)\(Ksp=(x)(2x)^2\)0.0184 M.Solubility productIn chemistry, when a solid is dissolved in water, one must take into account the fact that not necessarily its 100 % will be able to break into ions and thus undergo dissolution.
In such a way, and specially for sparingly soluble solids, one ought to write the dissolution reaction at equilibrium as shown below for the given barium fluoride:
\(BaF_2(s)\rightleftharpoons Ba^{2+}(aq)+2F^-(aq)\)
Next, we can write its equilibrium expression according to the law of mass action, which also demands us to omit any solid and refer it to the solubility product constant (Ksp):
\(Ksp=[Ba^{2+}][F^-]^2\)
Afterwards, one can insert the reaction extent, x, as it stands for the molar solubility of this solid in water, taking into account the coefficients balancing the reaction:
\(Ksp=(x)(2x)^2\)
Finally, we solve for the x as the molar solubility of barium fluoride as shown below:
\(2.5x10^{-5}=(x)(2x)^2\\\\2.5x10^{-5}=4x^3\\\\x=\sqrt[3]{\frac{2.5x10^{-5}}{4} } \\\\x=0.0184M\)
Learn more about chemical equilibrium: https://brainly.com/question/26453983
The atmospheric gas that forms a mild acid when dissolved in water is _____.
Answers
Answer:
Carbon dioxide
HAVE A GOOD DAY!
Answer:
Carbon Dioxide
Explanation:
Carbon dioxide is in the atmosphere and is an end product in organisms that obtain energy from breaking down sugars, fats and amino acids with oxygen as part of their metabolism.
draw the structure of iso -pentylpropanoate
Answers
Phenolphthalein turns litmus paper what color?
Answers
Litmus paper and Phenolphthalein are both indicators. This means they will change colour in the presence of an acid or a base.
Litmus paper turns blue in a base and remains red when it is in contact with an acid or neutral solution. Phenolphthalein turns pink in a base, but is coluorless in an acid or neutral solution.
which of the following correctly shows how to convert a density of 20.1 g cm-3 to units of kg m-3?20.1g/1 cm3 Times 1000 kg/1g Times 1cm3/0.01m3 20.1g/1cm3Times 1 kg/1000gTimes 1cm3/0.01m320.1g/1 cm3Times 1 kg/100 gTimes0.01 cm3/ m3 20.1 g/1 cm3Times1 kg/1000 gTimes (0.01 CM)3/(1 M)3 20.1 G/1 CM3Times 1 KG/1000g Times (1 cm)3/(0.01 m)3
Answers
The final result is 20100 kg/m³, which is the equivalent density in SI units. This means that a material with a density of 20.1 g/cm³ has a density of 20100 kg/m³ when expressed in SI units. It is important to use the correct units when working with scientific data to ensure that calculations are accurate and meaningful.
To convert a density of 20.1 g/cm³ to units of kg/m³, we need to use unit conversion factors that relate the two sets of units. We know that 1 cm³ is equivalent to 0.000001 m³, and 1 g is equivalent to 0.001 kg. Therefore, we can write:
20.1 g/cm³ x (0.001 kg/g) x (1 cm/0.01 m)^3 = 20100 kg/m³
In the first step, we multiply by the conversion factor of 0.001 kg/g to convert grams to kilograms. In the second step, we use the conversion factor of (1 cm/0.01 m)^3 to convert cm³ to m³. We raise the factor to the third power because we are dealing with volume, which is a cubic quantity.
The final result is 20100 kg/m³, which is the equivalent density in SI units. This means that a material with a density of 20.1 g/cm³ has a density of 20100 kg/m³ when expressed in SI units. It is important to use the correct units when working with scientific data to ensure that calculations are accurate and meaningful.
Learn more about density here:
https://brainly.com/question/15164682
#SPJ11
Calculate the pH after 0.13 mol NaOH are added to 1.00 L of 0.50M HC2H3O2 and 0.80M NaC2H3O2 buffer solution, Ka= 1.3 x 10^-5. I really need help, I'm struggling with this topic.
Answers
Determine how many mL of solution A (acetic acid-indicator solution) must be added to solution B (sodium acetate-indicator solution) to obtain a buffer solution that is equimolar in acetate and acetic acid. Solution A: 10.0 mL 3.0e-4M bromescol green
hoped I helped
The following equation is an example of a ______________ reaction. 2 NaCl + F2 → 2 NaF + Cl2 ???
Synthesis
Decomposition
Single replacement
Double replacement
Answers
Answer:
double replacement is the answer
Which of the following is a true statement?
Work requires only sound energy.
Energy can accomplish work.
Energy is not necessary to do work.
Work requires only light energy.
Answers
Answer:
b) energy can accomplish work
Explanation:
Hydrogen reacts explosively with oxygen. however, a mixture of and can exist indefinitely at room temperature. explain why and do not react under these conditions.
Answers
Under the circumstances, H2 and O2 do not react because not enough energy is used in the process.
What happens when hydrogen and oxygen are heated together?Energy is released and the molecules of hydrogen and oxygen can join to generate either water or hydrogen peroxide when the molecules of H2 and O2 are mixed and allowed to react.
Why can't H2O2 be kept in storage for a long time?Hydrogen peroxide is very reactive and unstable. At standard pressures and temperatures, its vapours have an explosive character. It quickly breaks down and may cause dangerous reactions. hence it needs to be kept with extreme care.
To know more about Hydrogen visit:-
https://brainly.com/question/5172490
#SPJ4
What was Robert Andrews Millikan atomic model??? and plz explain And if he has Explanation and definition of the models.
Answers
Explanation:
In 1909 millikan began a series of experiments to determine the electric charge carried by a single electron.He bagan by measuring the course of charged water droplets in an electric field......He found that all the drops had charges that were simple multiples of a single number, the fundamental charge of the electron.
Difference between Lewis Dot Diagrams and Lewis Structures
Answers
Answer:
The Lewis formalism used for the H2 molecule is H:H or H—H. The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond.
Explanation:
4.What system is the system that takes in oxygen and releases carbon dioxide.
skeletal
muscular
circulatory
respiratory
Answers
How many more valence electrons does chlorine need to satisfy the octet rule? Chlorine is in group 17
Answers
Answer:
1
Explanation:
Octet meaning 8. So if chlorine already has 7 valence electrons it will only need to get 1 more to be satisfied.
What was the factor that caused the widely different separations between the four medicinal compounds
Answers
The factor that caused the widely different separations between the four medicinal compounds is their different affinities for the stationary phase in the chromatographic technique used.
Chromatography is a separation technique that relies on the differential affinities of compounds between a mobile phase and a stationary phase. The stationary phase is a solid or liquid material that is immobilized on a support, while the mobile phase is a liquid or gas that moves through the stationary phase.
In chromatography, compounds with stronger affinity for the stationary phase will have slower movement and shorter distances traveled, while compounds with weaker affinity for the stationary phase will have faster movement and longer distances traveled.
The four medicinal compounds being separated in the chromatographic process possess different chemical properties, such as polarity, molecular size, and functional groups. These differences in chemical properties result in different interactions between the compounds and the stationary phase.
Compounds that have stronger interactions with the stationary phase, such as through hydrogen bonding or dipole-dipole interactions, will have slower movement and shorter separations. On the other hand, compounds with weaker interactions or higher solubility in the mobile phase will have faster movement and longer separations.
Therefore, the widely different separations observed between the four medicinal compounds can be attributed to their unique chemical properties, leading to varying degrees of affinity for the stationary phase in the chromatographic technique.
The differing chemical properties and affinities of the four medicinal compounds for the stationary phase caused the widely different separations observed in the chromatographic process.
To read more about chromatographic technique, visit:
https://brainly.com/question/28731153
#SPJ11