A btu is defined as the amount of energy required to raise the temperature of one lb of water by one degree celsius.
a. True
b. False
Answers
Answer:One BTU is defined as the amount of energy required to raise the temperature of 1 lb of water through 1 degree Fahrenheit
Explanation:
Related Questions
A scientist conducts experiments to test what temperature causes the fastest growth rate in sunflower plantsafter multiple trials the results show that sunflower plants grow the fastest at 75 FIs this experement an example of replication or repetition?
Answers
Answer:
Repetition
Explanation:
In the world of science, experiments are regularly conducted to test out hypotheses. These experiments are however, not conducted by scientist once to derive a result. They are rather conducted multiple times (repeated) to ascertain the accuracy i.e not as a result of random occurrence. The experiments that undergo multiple trials are said to undergo REPETITION.
This is the case of the scientist in this question, who is conducting an experiment to test what temperature causes the fastest growth rate in sunflower plants. He conducted the experiment multiple times, making it an example of REPETITION.
N.B: Replication is when the experiment is re-conducted by another scientist to see if same result is derived.
arrange the following in order of increasing Rf on thin layer chromatography: acetic acid, acetaldehyde, 2-octanone, decane, and 1butanol
Answers
Acetic acid is followed by 1-butanol, acetaldehyde, 2-octanone, and decane in ascending order of such Rf value.
Acetic acid: what is it?Acetic anhydride, ethylic hydrochloric, alcoholic acid, as well as carbon carboxylic acid are other names for acetic anhydride, which has the chemical formula CH3COOH. Vinegar has a characteristic scent that is caused by alcohol, a result of fermentation. Water makes up about 4-6percent of total of something like the acetic acid present in vinegar. Acetic acid is typically accepted to be safe to include in products if it is "food-grade."
What function does acetic acid serve?Acetic acid is the 33rd most frequently manufactured chemical in the US. Products manufactured from acetic acid include acetaldehyde, methacrylic acid, vinyl acetate gelation, acetic acids, chloracetic vinegar, plastic components, dyes, insecticides, photographic chemicals, and leather.
To know more about acetic acid visit:
https://brainly.com/question/16970860
#SPJ4
what is the answer PLEASE HELP!!

Answers
Answer:
the water displaced will not help the student find the correct volume because you can not accurately calculate it. you have to get the water when it is settled then you place the wood in.
Explanation:
4. They are the muscles which have complete control and responsible for all
kinds of body movement
A. Contracting B. Involuntary C. Relaxing D. Voluntary
5. What system that is made up of many bones joined to form a structure?
A. Digestive B. Integumentary C. Muscular
D. Skeletal
6. How do our muscles work?
D. By touching
A. By pulling B. By smelling C. By seeing
7. These are the long bones of our body.
A legs B. Patella
C. Ribs D. Tendons
8. What composes the lower extremities of the body?
A backbone B. Compact bone C. Knee bone D. Pelvic bone
9. An organ that manufactures the blood cells in our body?
A Bone marrow B. Compact bone C. Knee bone D. Pelvic bone
10. Are muscles that move without conscious effort like the beating of our heart.
A contracting B. Involuntary
C. Relaxing
D. Voluntary
Answers
Option B: voluntary muscles are the muscles which have complete control and are responsible for all kinds of movements.
Animals' muscles are the types of tissue that cause movement or motion. Voluntary refers to action taken of one's own free will or will. All of the muscles that are linked to the skeleton are voluntary muscles, hence these muscles are also referred to as skeletal muscles or striated muscles because of the way their muscle fibres give them a striated, or stripy, appearance. This, therefore, tells us that option B is he right choice.
The only muscles that can be actively manipulated are those in the skeleton. Since the muscles are connected to the bones, pulling on them moves the bones. Skeletal muscles are used for every conscious movement a person makes.
To know more about skeletal muscles, refer to the following link:
https://brainly.com/question/12252128
#SPJ4
What are the best titles a and b A science b pseudoscience
Answers
i don't really know what you are trying to say here, but what i think you are saying is what sounds like a more impressive job?? in my opinion, pseudoscience sounds more fancy and interesting. Since not a lot of people really knows what that is, it sparks conversation.
Answer:
https://brainly.in/question/5343031 use this link
Explanation:
According to the question;
A student takes notes in class as shown below. A. Uses an objective process Is updated constantly B. Is not objective Does not use a process Resists new information.
So the best titles for circumstances A and B will be :
Option C ;
A) characteristics of science because of the scientific methods used for notes taking
B) limitations of science because of the non scientific method or Science resistive methods used for notes taking
1: What is the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25∘
C ? A) 39,00 B) 39.10 C) 17.90 D) 19.55 E) 15200 Q2: Calculate the root mean square speed (m/s) of an A2 molecule (atomic mass =6amu ) at 0∘ C. A) 1506 B) 1.135×10 6
C) 1065.0 D) 1065.3 E) 753
Expert Answer
Answers
In a 2.50-L container with 56 g each of nitrogen and carbon dioxide gases added at 25°C, the partial pressure of nitrogen gas is 4.89 atm. The root mean square speed of an A₂ molecule at 0°C is approximately 8.65 m/s. None of the given options are correct.
Q1. We need to find the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C. Let's begin:
56g of N₂ will be equal to moles of N₂ = (56/28) = 2 mol N₂
56g of CO₂ will be equal to moles of CO₂ = (56/44) = 1.27 mol CO₂
Total moles of gas = 2 + 1.27 = 3.27 moles
Molar mass of N₂ = 28 g/mol
Ideal Gas equation = PV = nRT
Partial pressure of N₂ = P_N₂ = (n_N₂ * RT) / V
Where,
n_N₂ = number of moles of N₂ = 2 mol
R = gas constant = 0.08206 L atm K⁻¹ mol⁻¹
T = temperature in kelvin = (25 + 273.15) K = 298.15 K
V = volume of container = 2.50 L
Substituting the values in the formula, we get:
P_N₂ = (2 mol * 0.08206 L atm K⁻¹ mol⁻¹ * 298.15 K) / 2.50 L
P_N₂ = 4.89 atm
Therefore, the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C is 4.89 atm.
None of the given options are correct.
Q2. We need to find the root mean square speed (m/s) of an A₂ molecule (atomic mass =6amu ) at 0°C. Let's begin:
The root mean square speed (v rms) of an ideal gas is given by the formula:
v rms = √(3RT / M)
Where,
R = gas constant = 8.314 J/mol K
T = temperature in kelvin = 0 + 273.15 K = 273.15 K
M = molar mass of the gas = 6 g/mol = 6 / 1000 kg/mol = 0.006 kg/mol
Substituting the values in the formula, we get:
v rms = √(3 * 8.314 J/mol K * 273.15 K / 0.006 kg/mol)
v rms = √(3 * 8.314 J/mol K * 273.15 K / 6 * 10⁻³ kg/mol)
v rms = √(74.81)
v rms = 8.65 m/s (approx.)
Therefore, the root mean square speed (m/s) of an A₂ molecule (atomic mass =6amu ) at 0°C is 8.65 m/s (approx.).
None of the given options are correct.
To know more about root mean square speed, refer to the link below:
https://brainly.com/question/30759623#
#SPJ11
Complete Question:
Q24: What is the partial pressure (atm) of nitrogen gas in a 2.50-L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C ? A) 39.00 B) 39.10 C) 17.90 D) 19.55 B) 15200
Q25: Calculate the root mean square speed (m/s) of an A2 molecule (atomic mass =6amu) at 0°C. 4) 1506 B) 1.135×10⁶ C) 1065.0 D) 1065.3 E) 753
which one of the following properties is least characteristic of typical metals?a.moderately high melting pointb.high boiling pointc.brittlenessd.good electrical conductor when solide.good electrical conductor when molten
Answers
The property that is least characteristic of typical metals is brittleness (option c).
Metals are known for their malleability and ductility, meaning they can be easily shaped and stretched without breaking.
Therefore, they are not typically brittle. The other options, moderately high melting point (option a), high boiling point (option b), and good electrical conduct when solid or molten (options d and e), are all characteristics of typical metals.
Metals generally have high melting and boiling points compared to other materials. This is due to their unique atomic structure and the way that their metallic bonds operate.
In metals, the outer electrons of each atom are delocalized, meaning that they are free to move throughout the material. This creates a sea of electrons that holds the atoms together in a lattice structure. The strength of these metallic bonds contributes to the high melting and boiling points of metals.
Learn more about melting point : https://brainly.com/question/40140
#SPJ11
Answer the following questions in complete sentences.
A. Identify club soda as an element, compound, or mixture.
B. Explain the classification of club soda identified in Part A.

Answers
Answer:
a) club soda is a compound
b) Club soda is a manufactured form of carbonated water, commonly used as a drink mixer. Sodium bicarbonate, potassium sulfate, potassium bicarbonate, potassium citrate, or sodium citrate is artificially added to replicate constituents commonly found in natural mineral waters
How much heat is needed to warm 250g of water from 22℃ to 98℃?

Answers
Answer:
Thus, the required heat is 79kJ 79 k J .
Explanation:
i think its the right bottem one
Find how much mass is in 2.1 moles H2O
Answers
2.1 moles of \(H_2O\) will have a mass of 37.8 grams.
Moles and masses of substancesThe number of moles a substance contains and the mass of the substance are related by the following equation:
Mole = mass/molar mass
In other words, the mole of a substance is the ratio of the mass of the substance and its molar mass.
Rearranging the equation:
Mass = mole x molar mass.
In this case, we want to find the mass of 2.1 moles of water. The molar mass of water can be calculated as follows:
\(H_2O\) = (1 x 2) + 16
= 18 g/mol
Mass of 2.1 moles of water = 2.1 x 18
= 37.8 grams
In other words, 2.1 moles of water will have a mass of 37.8 grams.
More on moles of substances can be found here: https://brainly.com/question/26416088
#SPJ1
OSTOICHIOMETRY
Using molarity to find solute moles and solution volume
A chemist adds 440.0 mL of a 1.46M barium acetate
added to the flask. Round your answer to 3 significant digits.
mol
be (Ba(C₂H₂O₂),) solution to a reaction flask, Calculate the millimoles of barium acetate the chemist has
X
Calculator
542400
Maribel V
do
Answers
The chemist has 642.4 millimoles of barium acetate in the solution.
To calculate the millimoles of barium acetate (Ba(C₂H₃O₂)₂) in the solution, we can use the formula:
moles = molarity × volume (in liters)
First, let's convert the volume from milliliters (mL) to liters (L):
440.0 mL ÷ 1000 = 0.440 L
Now we can substitute the given values into the formula:
moles = 1.46 M × 0.440 L
moles = 0.6424 mol (rounded to 4 decimal places)
To convert the moles to millimoles, we multiply by 1000:
millimoles = 0.6424 mol × 1000
millimoles = 642.4 mmol (rounded to 3 significant digits)
Therefore, the chemist has 642.4 millimoles of barium acetate in the solution.
It's important to note that the molarity (M) represents the number of moles of solute per liter of solution. By multiplying the molarity by the volume in liters, we can find the number of moles of solute. To convert moles to millimoles, we multiply by 1000. The result represents the millimoles of barium acetate present in the given volume of solution.
For more such questions on barium acetate visit:
https://brainly.com/question/15304103
#SPJ8
The balanced chemical equation for the reaction between sodium chloride and silver nitrate is:
NaCl ( aq ) + AgNO3 ( aq ) AgCl ( s ) + NaNO3 ( aq )
We can interpret this to mean:
1 mole of sodium chloride and mole(s) of silver nitrate
React to produce:
mole(s) of silver chloride and mole(s) of sodium nitrate
Answers
Answer:
We can Interprete it as 1mole of Sodium Chloride and 1mole of Silver Nitrate React to Produce
1Mole of Silver Chloride and 1Mole of Sodium Nitrate
Answer:NaCL
Explanation:Edg
Determine the number of protons, neutrons, and electrons for a neutral potassium-41 isotope.
Answers
Atomic number is equal to the number of electrons in a neutral atom. ... Niobium has an atomic number of 41. ... Determine the number of protons, electrons, and neutrons for each isotope described ... Potassium-41. 19
Which number corresponds to the number in
scientific notation?
3.21 x 10^4 kg= kg
2.0 x 10^-5L= L
Answers
The number that corresponds to the numbers in scientific notation is as follows:
3.21 × 10⁴kg = 32100kg2.0 × 10-⁵L = 0.00002LWhat is scientific notation?Scientific notation is a method of writing, or of displaying real numbers as a decimal number between 1 and 10 followed by an integer power of 10.
It is an alternative format of such a decimal number immediately followed by E (exponential) and an integer. For example, the number 0.00236 can be written in scientific notation as 2.36 × 10-³ or as 2.36E-3.
In the case of the above example, 2.36 is the integer while E-03 is the exponential.
This means we can derive the number depicted by the scientific notation as given in the main answer part of this answer.
Learn more about scientific notation at: https://brainly.com/question/18073768
#SPJ1
Answer: Look at the picture below - Hope this helps <33
Explanation:

Why do we need units of pressure?
Answers
Answer:Because there are so many units for energy.
Explanation:
What did Neil Armstrong earn a master’s degree in from the University of Southern California?
Answers
Neil Armstrong earned a Master of Science degree in Aerospace Engineering from the University of Southern California in 1970.
He pursued this degree while working as a NASA astronaut His thesis was focused on the stability of a lunar landing vehicle during the final phase of descent.
In 1962, Armstrong joined NASA as a civilian test pilot and astronaut. He soon became part of the Gemini and Apollo space programs. During his tenure at NASA, he piloted the Gemini 8 mission in 1966. He also commanded the Apollo 11 mission in 1969, where he became the first person to set foot on the Moon.
Learn more about Neil Armstrong, here:
https://brainly.com/question/30763002
#SPJ1
what si the ratio of glyceraldehyde-3-phosphate gap to dihydroxyacetone phosphate
Answers
Glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) are two isomers of the glycolytic intermediate in cells. The ratio of GAP to DHAP is 1:1 at equilibrium conditions, but it varies significantly under nonequilibrium conditions, such as in the presence of enzymes.
Under such conditions, the GAP to DHAP dihydroxyacetone phosphate ratio can vary between 3:1 and 20:1.The two molecules, GAP and DHAP, interconvert rapidly, and thus, they exist in a rapid equilibrium in cells. In cells, DHAP is an intermediate in glycolysis, and it is converted to GAP by the enzyme triose phosphate isomerase (TPI). The interconversion of GAP to DHAP by TPI is a reversible reaction and is known to be near-equilibrium. However, in glycolysis, the DHAP is typically rapidly utilized by an enzyme called aldolase, such that the DHAP concentration remains low relative to the GAP concentration, which accumulates. Therefore, the ratio of [GAP]:[DHAP] is typically greater than 1:1 under nonequilibrium conditions.Glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) are two isomers of the glycolytic intermediate in cells. The ratio of GAP to DHAP is 1:1 at equilibrium conditions, but it varies significantly under nonequilibrium conditions, such as in the presence of enzymes. Under such conditions, the GAP to DHAP ratio can vary between 3:1 and 20:1.The two molecules, GAP and DHAP, interconvert rapidly, and thus, they exist in a rapid equilibrium in cells. In cells, DHAP is an intermediate in glycolysis, and it is converted to GAP by the enzyme triose phosphate isomerase (TPI). The interconversion of GAP to DHAP by TPI is a reversible reaction and is known to be near-equilibrium. However, in glycolysis, the DHAP is typically rapidly utilized by an enzyme called aldolase, such that the DHAP concentration remains low relative to the GAP concentration, which accumulates. Therefore, the ratio of [GAP]:[DHAP] is typically greater than 1:1 under nonequilibrium conditions.
learn more about dihydroxyacetone phosphate Refer: https://brainly.com/question/13077675
#SPJ11
complete question: What is the ratio of glyceraldehyde-3-phosphate (GAP) to dihydroxyacetone phosphate (DHAP) in cells at 37 ∘ C under nonequilibrium conditions? [GAP]:[DHAP]= __:1
The reaction between a strong acid and a weak base produces a salt, but water is not usually formed because:
the reaction is too hot and water evaporates
there is no hydrogen present to form water
the acid is not strong enough to form water
weak bases tend not to be hydroxides
Answers
Answer:
3
Explanation:
I'll give brainless! no l!nks!!!
1. Imagine a very warm 60 degree day in January with a steady rain. What type of air mass would you expect to be bringing in this weather pattern?
2. Imagine a very cold 20 degree day in January with lots of blue sunny skies. What type of air mass would you expect to be bringing in this weather pattern?
Answers
Answer:
Maritime tropical air mass
Continental polar air mass
HELP FAST!
Which statement best describes how energy is transformed during photosynthesis? A. plants take in chemical energy and change it to heat energy B. plants take in light energy and change it to chemical energy in the form of food C. plants take in heat energy and change it to light energy D. plants take in chemical energy and change it to light energy
Answers
Answer:D
Explanation:
The statement best describes how energy is transformed during photosynthesis is the plants take in chemical energy and change it to light energy. Therefore, option D is correct.
What is photosynthesis ?Photosynthesis is a process that plants and other organisms used to convert light energy into chemical energy that can then be released to fuel the organism's activities via cellular respiration.
Photosynthesis accounts for nearly all the oxygen in the atmosphere. If photosynthesis were to cease, there would soon be little food or other organic matter on Earth, most organisms would vanish, and the Earth's atmosphere would eventually be nearly devoid of gaseous oxygen.
Photosynthesis is the process by which plants absorb chemical energy and convert it to light energy.
Thus, option D is correct.
To learn more about the photosynthesis, follow the link;
https://brainly.com/question/29775046
#SPJ6
Why do animals eyes like dogs or cats sometimes glow when you take photos?
Answers
I need help in practice 31 trying to find out the name of the hydrocarbon compound

Answers
The hydrocarbon compound with the formula CH₃ C(CH₃)₂ CH(CH₃) CH₂ CH₂ CH₃ is 2,2-dimethylpentane.
How to name hydrocarbons?To name a hydrocarbon, identify the longest continuous chain of carbon atoms. In this case, the longest chain has 5 carbon atoms. This tells you that the compound is a pentane.
Next, identify any branches off of the main chain. In this case, there are two branches, each of which has 2 carbon atoms. These are called methyl groups.
Finally, number the carbon atoms in the main chain so that the branches are attached to the lowest possible number. In this case, the carbon atoms are numbered 1, 2, 3, 4, 5, and 6. The branches are attached to carbon atoms 2 and 4.
The name of the compound is then 2,2-dimethylpentane. The "2,2" tells you that the methyl groups are attached to carbon atoms 2 and 2. The "dimethyl" tells you that there are two methyl groups. The "pentane" tells you that there are 5 carbon atoms in the main chain.
Find out more on hydrocarbon compound here: https://brainly.com/question/30363912
#SPJ1
The object of a general chemistry experiment is to determine the amount (in millilitres [mL]) of sodium hydroxide (NaOH) solution needed to neutralize 1 g of a specified acid. This will be an exact amount, but when the experiment is run in the laboratory, variation will occur as the result of experimental error. Three titrations are made using phenolphthalein as an indicator of the neutrality of the solution (pH equals 7 for a neutral solution). The three volumes of NaOH required to attain a pH of 7 in each of the three titrations are as follows: 82.16, 75.79, and 75.43 mL. Use a 99% confidence interval to estimate the mean number of millilitres required to neutralize 1 g of the acid. (Round your answers to three decimal places.)
to mL
Answers
The answer is (70.35, 84.57) mL.
The three volumes of NaOH required to attain a pH of 7 in each of the three titrations are: 82.16, 75.79, and 75.43 mL. To estimate the mean number of milliliters required to neutralize 1 gram of the acid, a 99 percent confidence interval will be used. Let's calculate the sample mean, sample standard deviation, and margin of error using the provided data.
Sample standard deviation:
Sample Mean The sample mean of a dataset is defined as the sum of all the data points divided by the number of data points. So the sample mean will be: (82.16+75.79+75.43) / 3 = 77.46 mL. Sample Standard Deviation The sample standard deviation (s) is defined as the square root of the sample variance. To calculate s, we need to first compute the sample variance (s²):s² = ∑(x - μ)² / (n - 1)where x is the value of the observation, μ is the sample mean, and n is the sample size.s² = [(82.16 - 77.46)² + (75.79 - 77.46)² + (75.43 - 77.46)²] / (3 - 1)s² = [20.4 + 6.74 + 5.84] / 2s² = 16.49s = sqrt(16.49) = 4.06 mL.
Marginal Error The formula for the margin of error for a confidence interval for the mean is:
margin of error = t (α/2) * (s / sqrt(n)) where t(α/2) is the critical value of the t-distribution with n-1 degrees of freedom and a level of significance of α/2 (in this case, α/2 = 0.005).s is the sample standard deviation that we computed earlier. n is the sample size (in this case, n = 3). margin of error = t(α/2) * (s / sqrt(n))margin of error = 3.182 * (4.06 / sqrt(3)) = 7.11 mL. The margin of error is 7.11 mL. Confidence Interval The confidence interval formula for a population mean is: sample mean - margin of error < μ < sample mean + margin of error where μ is the population mean and sample mean is the value obtained from the sample.μ = 77.46 - 7.11 < μ < 77.46 + 7.11Thus, the 99% confidence interval for the mean number of milliliters needed to neutralize 1 gram of the acid is (70.35, 84.57) mL (rounded to three decimal places).Therefore, the answer is (70.35, 84.57) mL.
Read more about standard deviation at; brainly.com/question/475676
#SPJ11
Between __________ of U.S. adults over age 40 have diverticular disease. Roughly __________ of individuals with diverticular disease show signs and symptoms of the disease. The percentage of U.S. adults having the disease jumps to 50% by age of 60.
Answers
Diverticular disease is a common condition among adults over the age of 40 in the United States. Studies suggest that between 10% to 40% of U.S. adults over 40 have diverticular disease. This condition occurs when small, bulging pouches (diverticula) develop in the lining of the digestive system, most commonly in the large intestine.
Symptoms of diverticular disease can vary, but roughly 15% to 25% of individuals with diverticular disease show signs and symptoms of the disease, which may include abdominal pain, bloating, constipation, and diarrhea. The likelihood of developing diverticular disease increases with age, and the percentage of U.S. adults having the disease jumps to 50% by the age of 60. Other risk factors for developing diverticular disease include a diet low in fiber, obesity, smoking, lack of physical activity, and certain medications.
If left untreated, diverticular disease can lead to complications such as infections, bleeding, and perforations in the intestinal wall. Therefore, it is important to seek medical attention if you experience any symptoms of diverticular disease. Treatment may include dietary changes, medication, and, in severe cases, surgery.
Learn more about digestive system here-
https://brainly.com/question/29124378
#SPJ11
What happens to an atom that experiences radioactive decay?
O A. It loses mass.
B. It absorbs energy.
C. It gains electrons.
D. It burns up.
Answers
Answer:
I think the answer is a
Explanation:
An atom loses mass when the atom experiences radioactive decay.
What is radioactive decay?
Radioactive decay is when the balance of protons and neutrons in the nucleus isn't quite right, so it emits particles and energy.
Radioactive decay is when the nucleus of an atom isn't stable - it could have too many protons that push each other apart, or too many neutrons, and it's just like a big lump of rock and can fall apart.
There are three kinds of radioactive decay, all named after Greek letters: alpha (α), beta (β) and gamma (γ).
α-decay happens in unstable nuclei and an α-particle is emitted, which is equivalent to the helium nucleus. Two protons and two neutrons are emitted, reducing the total mass number by four and the atomic number by two, making the atom into a new, smaller, more stable element.β-decay is when you've got too many neutrons, so a neutron decays into a proton. γ-decay is where the atom emits a photon with the wavelength of a γ-ray.Hence, option A is the correct answer.
Learn more about radioactive decay here:
https://brainly.com/question/1770619
#SPJ2
Which of the following is not a magnetic material A cobalt B iron C nickel D plastic E steel
Answers
Answer:
D. plastic\(\tt{ \green{P} \orange{s} \red{y} \blue{x} \pink{c} \purple{h} \green{i} e}\)
calculate e.e. of a mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of ( )-glyceraldehyde.
Answers
The enantiomeric excess (e.e.) of the mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of (+)-glyceraldehyde is 48%.
To calculate the enantiomeric excess (e.e.) of a mixture, we need to determine the difference in the amounts of the two enantiomers and express it as a percentage of the total amount.
Given;
Mass of (-)-glyceraldehyde = 5.70 g
Mass of (+)-glyceraldehyde = 2.0 g
Calculate the total amount of glyceraldehyde in the mixture.
Total mass of glyceraldehyde = Mass of (-)-glyceraldehyde + Mass of (+)-glyceraldehyde
= 5.70 g + 2.0 g
= 7.70 g
Calculate the individual amounts of each enantiomer as a fraction of the total amount.
Fraction of (-)-glyceraldehyde = Mass of (-)-glyceraldehyde / Total mass of glyceraldehyde
= 5.70 g / 7.70 g
= 0.740
Fraction of (+)-glyceraldehyde = Mass of (+)-glyceraldehyde / Total mass of glyceraldehyde
= 2.0 g / 7.70 g
= 0.260
Calculate the enantiomeric excess (e.e.).
e.e. = (Fraction of (-)-glyceraldehyde - Fraction of (+)-glyceraldehyde) × 100%
= (0.740 - 0.260) × 100%
= 0.480 × 100%
= 48%
Therefore, the enantiomeric excess (e.e.) of the mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of (+)-glyceraldehyde is 48%.
To know more about enantiomeric excess here
https://brainly.com/question/32199328
#SPJ4
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.
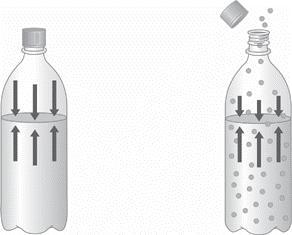

Answers
Answer:
SEE EXPLANATION
Explanation:
If i refer to the bottle at the left hand side as A and the bottle at the right hand side as B. I can say that the bottle A contains solutes which are more soluble than the solutes in bottle B. consequently, A has a lower vapour pressure than B.
We can also see that the pressure in A is much higher than in B(as indicated by the length of the arrows). The higher the pressure, the greater the solubility of the gas. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Again let me call the beaker on the left A and the beaker on the right B. We can see that the beaker on the left contains 10 ml of solution while the beaker on the right contains 50 ml of solution. It follows that beaker A contains a more concentrated solution since that much amount of solute is contained in a smaller volume than in beaker B as shown in the image.
can someone help me?

Answers
Answer:
1: because phosphate contain oxide ions so that is base character
a ground state hydrogen atom absorbs a photon of light having a wavelength of 97.2 nm. it then gives off a photon having a wavelength of 486 nm. what is the final state of the hydrogen atom?
Answers
After taking in a photon with a wavelength of 97.2 nm, the hydrogen atom moves into the n = 3 energy level.
Has hydrogen got one or two atoms?The nucleus of the hydrogen atom is made up of a single proton and an electron. Two hydrogen atoms are linked together to form the hydrogen molecule. Hydrogen has a molar mass of 2.016 g/mol.
Using the following formula to determine a photon's energy:
E = hc/λ
where:
h = Planck's constant = 6.626 x 10⁻³⁴J.s
c = speed of light = 2.998 x 10⁸ m/s
λ = wavelength in meters
One can compute the energy of the absorbed photon as follows:
E = hc/λ = (6.626 x 10⁻³⁴ J.s) x (2.998 x 10⁸m/s) / (97.2 x 10⁻⁹ m) = 2.042 x 10⁻¹⁸ J
The following equation can be used to determine the energy levels of hydrogen:
En = -13.6 eV / n²
where:
En = energy of the nth energy level in electron volts (eV)
We may calculate the final energy level by equating the energy difference to the difference between two energy levels:
2.042 x 10⁻¹⁸ J = (-13.6 eV / nf²) - (-13.6 eV / ni²)
where:
nf = final energy level
ni = initial energy level (which is 1 for the ground state)
Solving for nf, we get:
nf = sqrt(13.6 eV / (13.6 eV / 1² - 2.042 x 10⁻¹⁸ J)) = 3
To know more about hydrogen atom visit:-
https://brainly.com/question/30886690
#SPJ1