Answers
CCC Pattern compare the ions of elements listed in the table with the atoms of the element. Based on the patterns in their particle compositions and charges, the definition of ion would be that CCC pattern compare ions of elements.
An ion is a structure in crystal system having regular repeating arrangement, which can be called as an ionic lattice.
Lattice is formed because ions attract each other and form a regular pattern with oppositely charged ion next to each other.
An atom is the smallest unit of an element that retains the chemical properties of element. Every atom includes a nucleus, generally containing both positively charged particles called ions and uncharged particles called ion.
In the space surrounding, the nucleus are negatively charged particles called electron.
Therefore, ions are oppositely charged particles in lattice.
To learn more about CCC patterns,
brainly.com/question/24741756
#SPJ1
Related Questions
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
By which method the following mixture can be separated?(i)mixture of colours (ii) Minerals oil.
Answers
the answer ie the second option
Minerals oil
(a) How many stereoisomers are possible for 4-methyl-1,2-cyclohexanediol? ___ (b) Name the stereoisomers formed by oxidation of (S)-4-methylcyclohexene with osmium tetroxide. If there is only one stereoisomer formed, leave the second space blank. Isomer #1: Isomer #2: (c) Is the product formed in step (b) optically active? _____
Answers
Answer:
See explanation
Explanation:
For the first part of the question, we have to check the chiral carbons in 4-methyl-1,2-cyclohexanediol. In this case carbons, 1 and 2 are chiral, if we have 2 chiral carbons we will have 4 isomers. We have to remember that formula 2^n in which "n" is the number of chiral carbons, so:
2^n = 2^2 = 4 isomers
And the isomers that we can have are:
1) (1R,2S)-4-methylcyclohexane-1,2-diol
2) (1S,2S)-4-methylcyclohexane-1,2-diol
3) (1S,2S)-4-methylcyclohexane-1,2-diol
4) (1S,2R)-4-methylcyclohexane-1,2-diol
See figure 1
For the second part of the question, we have to remember that the oxidation with \(OsO_4\) is a syn addition. In other words, the "OHs" are added in the same plane. In this case, we have the methyl group with a wedge bond, so the "OH" groups will have a dashed bond due to the steric hindrance. Due to this we only can have 1 isomer ((1S,2R,4S)-4-methylcyclohexane-1,2-diol). Finally, on this molecule, we dont have any symmetry planes (this characteristic will cancel out the optical activity), so the product of this reaction has optical activity.
See figure 2
I hope it helps!


Which of these is NOT a factor considered is
describing the climate of a region?
Answers
Answer:
the population of the region
Hope this helps :)
A pharmacist needs to make a 25.0 liter of a 3.50 M solution of a substance. The stock solution of the substance is 10.5 M. How much stock and water must be mixed?
1.47 liters of stock and 16.67 liters of water
8.33 liters of stock and 16.67 liters of water
1.47 liters of stock and 25.0 liters of water
8.33 liters of stock and 25.0 liters of water
Answers
The correct answer is option (b): 8.33 liters of stock and 16.67 liters of water. . The remaining volume will be filled with water, which is 25.0 L - 8.33 L = 16.67 L.
What is Solution?
A solution is a homogeneous mixture composed of two or more substances, where one substance (the solute) is uniformly dispersed in another substance (the solvent) at the molecular or ionic level. In a solution, the particles of the solute are evenly distributed throughout the solvent, resulting in a clear and uniform mixture.
To make a 25.0 liter solution of 3.50 M concentration, using a stock solution of 10.5 M, we can use the dilution formula:
\(C_{1}\) \(V_{1}\)=\(C_{2}\)\(V_{2\)
\(C_{1}\) = 10.5 M
\(V_{1}\) = volume of stock solution (unknown)
\(C_{2}\) = 3.50 M
\(V_{2\) = 25.0 L
Rearranging the formula to solve for \(V_{1\):
\(V_{1}\) = (\(C_{2}\)\(V_{2\)) / \(C_{1}\)
\(V_{1}\)= (3.50 M)(25.0 L) / 10.5 M
\(V_{1}\)= 8.33 L
So, the pharmacist needs to mix 8.33 liters of the 10.5 M stock solution with water to make a 25.0 liter solution of 3.50 M concentration. The remaining volume will be filled with water, which is 25.0 L - 8.33 L = 16.67 L.
Learn more about Solution from the given link
https://brainly.com/question/25326161
#SPJ1
What was the purpose of the experiment of cellular respiration
Answers
Answer:
Cellular respiration is used to create usable energy from the foods that living things eat. It's important to know that the reactions involved in cellular respiration are catabolic, meaning they break down molecules into smaller ones. This differs from anabolic reactions, which build bigger molecules from smaller ones
In Part A, we saw that the theoretical yield of aluminum oxide is 0.700 mol . Calculate the percent yield if the actual yield of aluminum oxide is 0.532 mol .
Answers
Considering the definition of percent yield, the percent yield is 76%.
Percent yieldThe percent yield is the ratio of the actual return to the theoretical return expressed as a percentage.
The percent yield is calculated as the experimental yield divided by the theoretical yield multiplied by 100%:
\(percent yield=\frac{actual yield}{theorical yield}x100\)
where the theoretical yield is the amount of product acquired through the complete conversion of all reagents in the final product, that is, it is the maximum amount of product that could be formed from the given amounts of reagents.
Percent yield in this caseIn this case, you know:
actual yield= 0.532 molestheorical yield= 0.700 molesReplacing in the definition of percent yield:
\(percent yield=\frac{0.532 moles}{0.700 moles}x100\)
Solving:
percent yield= 76%
Finally, the percent yield is 76%.
Learn more about percent yield:
brainly.com/question/14408642
#SPJ1
White light can be separated into a spectrum
of colors because each color of light
Answers
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
Calculate the percent composition of H3PO4
Answers
The percent composition of hydrogen, phosphorous and oxygen in phosphoric acid is 3.06 %,31.60%,65.31 % respectively.
What is percent composition?Percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates solute to solvent as,mass of solute/mass of solution ×100.There are two types of percentage composition percent weight by volume and percent volume by volume .
Percent composition of hydrogen=3/97.99×100=3.06%.
Percent composition of phosphorous=30.97/97.99×100=31.60%
Percent composition of oxygen=64/97.99×100=65.31%
Learn more about percent composition,here:
https://brainly.com/question/17505281
#SPJ1
In a redox reaction, what role does the reducing agent play?
A. It gives up electrons
B. It keeps its electrons
C. It takes electrons
D. It takes oxygen atoms
Answers
Answer:
(A) and (D)is the correct answer
Explanation:
(A) in terms of electronic concept and (D) in terms of classical concept
coz you know
hope, it helps.......
Answer:
A. It gives up electrons
Explanation:
on PLATO
I got 5 out of 5 on quiz
match each level of protein structure with the covalent/non-covalent interaction or entropic driving force that is featured in its construction.
Answers
Primary -- covalent/peptide bonds, Secondary --- Hydrogen bonds, Tertiary -- Hydrophobic effect, Quaternary -- Disulfide bonds.
A covalent bond is created among two or more atoms by the mutual sharing with one or more electron pairs. The 2 different atomic nuclei lure these charged particles at the same time. When the thing that is different in electronegativity values of two or more atoms is too slight for transfer of electrons to form ions, a polar covalent forms. A hydrogen bond (or H-bond) in science is a mainly electrostatic attraction between a hydrogen (H) atom tightly attached to an more electronegative "donor" atom or group (Dn) and then another electronegative atom carrying a solitary pair of electrons—the hydrogen bond acceptor (Ac). The hydrophobic effect relates to non-polar molecules and single - molecule sections in a water - soluble solution's tendency to avoid making interaction with molecules of water.
Learn more about covalent bond here:
https://brainly.com/question/11674395
#SPJ4
How did he show that these particles had a charge on them?
Answers
J.J. Thomson discovered electrons and their negative charge through the cathode ray experiment, leading to the development of the plum pudding model of the atom.
J.J. Thomson, a British physicist, was the first to discover electrons in 1897.
He conducted the cathode ray experiment to identify the negatively charged particles.
The cathode ray tube is a vacuum-sealed glass tube with two electrodes at each end: a cathode and an anode.
When a high voltage electrical current is applied to the electrodes, the tube glows, indicating that the cathode rays are being emitted from the cathode and traveling through the tube towards the anode.
The cathode rays were found to have a negative charge, according to Thomson.
These rays were identified as particles by the presence of a magnet, which caused the particles to bend in the direction opposite to the magnet's polarity.
This discovery indicated that the particles had a charge on them because they were deflected by the magnetic field, which is only possible if the particles have an electric charge.
Thomson further concluded that these particles were about 1,000 times smaller than hydrogen atoms because of the degree of deflection they experienced in the magnetic field.
Furthermore, Thomson created the plum pudding model of an atom, in which electrons are dispersed throughout a positively charged matrix, based on his findings.
For more such questions on electrons
https://brainly.com/question/26084288
#SPJ8
How old is a piece of wood that has 0.7813% of the carbon-14 of a living organism
Answers
Answer:
About 21*.3 years old
Explanation:
A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (heat capacity of solid = 2.96 J/g・°C; heat capacity of liquid = 1.75 J/g・°C; ∆Hfus = 8.04 kJ/mol; Tfinal = 17.6 °C)
a) How much energy (in kJ) is absorbed/released to heat the solid?
b)How much energy (in kJ) is absorbed/released to melt the solid?
c)How much energy (in kJ) is absorbed/released to heat the liquid?
d) What is the total amount of energy that must be absorbed/released for the entire process?
Answers
Answer:
a) Q₁ = 58.82 KJ
b) Q₂ = 42.48 KJ
c) Q₃ = 29.22 KJ
d) Q = 130.52 KJ
Explanation:
a)
In order to find the energy absorbed to heat the solid, we will use:
\(Q_{1} = mC_{1}\Delta T_{1}\)
where,
Q₁ = Heat absorbed for heating solid = ?
m = mass of solid = 488.3 g = 0.4883 kg
C₁ = Specific Heat Capacity of Solid = 2.96 J/g °C
ΔT₁ = Change in temperature of Solid = Melting Temperature - Initial Temp.
ΔT₁ = 17.6°C - (-23.1°C) = 40.7°C
Therefore,
\(Q_{1} = (488.3\ g)(2.96\ J/g\ ^{0}C)(40.7\ ^{0}C)\)
Q₁ = 58.82 KJ
b)
In order to find the absorbed to melt the solid at 17.6°C, we will use:
\(Q_{2} = nH_{fus}\)
where,
Q₂ = Heat absorbed for melting solid = ?
H_fus = Heat of Fusion = 8.04 KJ/mol
n = no. of moles = \(\frac{m}{MM} = \frac{488.3\ g}{92.41\ g/mol} = 5.28 mol\)
Therefore,
\(Q_{2} = (5.28\ mol)(8.04\ KJ/mol)\)
Q₂ = 42.48 KJ
c)
In order to find the energy absorbed to heat the liquid, we will use:
\(Q_{3} = m C_{3}\Delta T_{3}\)
where,
Q₃ = Heat absorbed for heating Liquid = ?
m = mass of solid = 488.3 g = 0.4883 kg
C₃ = Specific Heat Capacity of Liquid = 1.75 J/g °C
ΔT₃ = Change in temperature of Liquid = Final Temp. - Melting Temp.
ΔT₃ = 51.8°C - 17.6°C = 34.2°C
Therefore,
\(Q_{3} = (488.3\ g)(1.75\ J/g\ ^{0}C)(34.2\ ^{0}C)\)
Q₃ = 29.22 KJ
d)
Total amount of energy absorbed during entire process is:
\(Q = Q_{1} + Q_{2} + Q_{3}\)
\(Q = 58.82\ KJ + 42.48\ KJ + 29.22\ KJ\)
Q = 130.52 KJ
In order to heat a 488.3 g solid, 58.8 kJ are required. To melt the solid, 42.5 kJ are required. To heat the liquid, 29.2 kJ are required. The total amount of energy absorbed is 130.5 kJ.
Initially, a 488.3 g solid at -23.1 °C is heated up to 17.6 °C (melting point). We can calculate the heat required (Q₁) using the following expression.
\(Q_1 = c \times m \times \Delta T = \frac{2.96J}{g.\° C } \times 488.3g \times (17.6\° C-(-23.1\° C)) \times \frac{1kJ}{1000J} = 58.8 kJ\)
where,
c: heat capacity of the solidm: massΔT: change in the temperatureAt 17.6 °C, we can calculate the heat (Q₂) required to melt the solid using the following expression.
\(Q_2 = \Delta H_{fus} \times \frac{m}{MM} = 8.04 kJ/mol \times \frac{488.3 g}{92.41g/mol} = 42.5kJ\)
where,
∆Hfus: enthalpy of fusionm: massMM: molar massThe liquid is heated from 17.6 °C to 51.8 °C. We can calculate the heat required (Q₃) using the following expression.
\(Q_3 = c \times m \times \Delta T = \frac{1.75J}{g.\° C } \times 488.3g \times (51.8\° C-17.6\° C)) \times \frac{1kJ}{1000J} = 29.2 kJ\)
c: heat capacity of the liquidm: massΔT: change in the temperatureThe total amount of energy absorbed (Q) is the sum of the energy absorbed in each step.
\(Q = Q_1 + Q_2 + Q_3 = 58.8kJ+42.5kJ+29.2kJ= 130.5kJ\)
In order to heat a 488.3 g solid, 58.8 kJ are required. To melt the solid, 42.5 kJ are required. To heat the liquid, 29.2 kJ are required. The total amount of energy absorbed is 130.5 kJ.
Learn more: https://brainly.com/question/10481356

Lithium is located in the first group of the periodic table. How many valence electrons does the element lithium have?
1
2
4
8
Answers
Answer: 1
Explanation: Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron.
1 is correct
for brainliest
Which statement best describes how the tilt of Earth's rotational axis relates to the amount of solar energy received?
A. When a hemisphere is tilting away from the sun, the whole planet receives less direct sunlight,
B. When a hemisphere is tilting toward the sun, that hemisphere receives less direct sunlight.
C. When a hemisphere is tilting toward the sun, the whole planet receives less direct sunlight.
D. When a hemisphere is tilting away from the sun, that hemisphere receives less direct sunlight.
Answers
Explanation:
A. Around December 21, the Northern Hemisphere tilts the farthest away from the Sun. This is called the northern winter solstice, and it is when we have the least amount of daylight of any time of the year.
B.Solstices and shifting solar declinations are a result of Earth's 23.5° axial tilt as it orbits the sun. Throughout the year, this means that either the Northern or Southern Hemisphere is tilted toward the sun and receives the maximum intensity of the sun's rays.
C.The tilt of the Earth's axis also defines the length of daylight. Daylight hours are shortest in each hemisphere's winter. Between summer and winter solstice, the number of daylight hours decreases, and the rate of decrease is larger the higher the latitude. The fewer sunlight hours the colder the nights
D.The second solstice occurs on December 21 or 22 when the north pole is tilting 23.5 degrees away from our Sun and the south pole is inclined toward it. This is the shortest day of the year in the northern hemisphere — the northern hemisphere winter solstice.
Nitrogen gas reacts with hydrogen gas to produce ammonia. How many liters of hydrogen gas at 95kPa and 15∘C are required to produce 100 g of ammonia
Answers
Answer:
222.30 L
Explanation:
We'll begin by calculating the number of mole in 100 g of ammonia (NH₃). This can be obtained as follow:
Mass of NH₃ = 100 g
Molar mass of NH₃ = 14 + (3×1)
= 14 + 3
= 17 g/mol
Mole of NH₃ =?
Mole = mass /molar mass
Mole of NH₃ = 100 / 17
Mole of NH₃ = 5.88 moles
Next, we shall determine the number of mole of Hydrogen needed to produce 5.88 moles of NH₃. This can be obtained as follow:
N₂ + 3H₂ —> 2NH₃
From the balanced equation above,
3 moles of H₂ reacted to produce 2 moles NH₃.
Therefore, Xmol of H₂ is required to p 5.88 moles of NH₃ i.e
Xmol of H₂ = (3 × 5.88)/2
Xmol of H₂ = 8.82 moles
Finally, we shall determine the volume (in litre) of Hydrogen needed to produce 100 g (i.e 5.88 moles) of NH₃. This can be obtained as follow:
Pressure (P) = 95 KPa
Temperature (T) = 15 °C = 15 + 273 = 288 K
Number of mole of H₂ (n) = 8.82 moles
Gas constant (R) = 8.314 KPa.L/Kmol
Volume (V) =?
PV = nRT
95 × V = 8.82 × 8.314 × 288
95 × V = 21118.89024
Divide both side by 95
V = 21118.89024 / 95
V = 222.30 L
Thus the volume of Hydrogen needed for the reaction is 222.30 L
A.) A student titrated a 15.00-mL sample of a solution containing a weak, monoprotic acid with NaOH. If the titration required 17.73 mL of 0.1036 M NaOH to reach the equivalence point, calculate the concentration (in M) of the weak acid in the sample.
B.) If the sample solution described in A). contained 0.1845 g of the Weak Acid, calculate the molar mass (in g/mol) of the weak acid.
Answers
Answer:
A) 0.1225 M
B) 100.4 g/mol
Explanation:
Step 1: Write the generic neutralization reaction
HA(aq) + NaOH(aq) ⇒ NaA(aq) + H₂O(l)
Step 2: Calculate the reacting moles of NaOH
17.73 mL of 0.1036 M NaOH react. The reacting moles are:
0.01773 L × 0.1036 mol/L = 1.837 × 10⁻³ mol
Step 3: Calculate the reacting moles of HA
The molar ratio of HA to NaOH is 1:1. The reacting moles of HA are 1/1 × 1.837 × 10⁻³ mol = 1.837 × 10⁻³ mol.
Step 4: Calculate the molar concentration of HA
1.837 × 10⁻³ moles of HA are in a 15.00 mL volume. The molar concentration is:
M = 1.837 × 10⁻³ mol / 0.01500 L = 0.1225 M
Step 5: Calculate the molar mass of HA
1.837 × 10⁻³ moles of HA weigh 0.1845 g. The molar mass of HA is:
0.1845 g / 1.837 × 10⁻³ mol = 100.4 g/mol
Calculate the number of moles of C2H6 in 3.97×1023 molecules of C2H6.
Answers
3.97×1023 molecules C2H6 1 mol C2H6
------------------------------------------ x ------------------------------------ = 0.66 mol C2H6
6.022 x 1023 molec. C2H6
Please help me with this reaction

Answers
The major product from the above organic reaction is I
In this reaction, methoxy ethane undergoes addition reaction with hydrogen bromide, Markownikoff's rule being involved in the reaction.
What are organic compounds?Organic compounds are the compounds which contains carbon and hydrogen.
Generally, organic compounds are characterized by the following:
All organic compounds contains carbon Organic compounds are also combustible in nature They are mostly covalent bonded molecules They are all soluble in non-polar solvents.They can be isolatedThey can also be prepared in the laboratoryBelow are some classes of organic compounds:
AlkanesAlkenesAlkynesAlkanolsAlkanoic acidAlkanalsEstersKetonesAminesSo therefore, the major product from the the above organic reaction is IV
Learn more about organic compounds:
https://brainly.com/question/704297
#SPJ1
What is true of spontaneous reactions?
O They are indicated by a negative change in Gibbs free energy.
O They have a positive value of AS.
O They are instantaneous.
O They always release heat.
Help 20pts
Answers
Explanation: Spontaneous reactions are those that occur without any external input of energy. A negative change in Gibbs free energy (ΔG) indicates that a reaction is spontaneous. The other options do not always hold true for spontaneous reactions. The value of entropy change (ΔS) can be positive or negative, spontaneous reactions are not necessarily instantaneous, and they do not always release heat.
Describe all the state of change that solid caffeine passes through in order to become gas?
Answers
The states change that solid caffeine passes through in order to become gas is ; Solid caffeine --- > liquid form -----> solid form ----->gaseous form
Caffeine is an alkaloid that is slightly soluble in hot water but we make use of dichloromethane because of the solubility of caffeine in an organic medium whereby the solid caffeine is converted to liquid.
next step is the use of anhydrous solids to remove the water component of the solution to get back the solid form of the caffeine. the organic solvent is evaporated, to purify the new solid form of caffeine.
Finally the solid form of caffeine undergoes sublimation ( changes from solid to gas ) to obtain a purer solid caffeine.
The solid caffeine undergoes ;
dissolution ; conversion of solid caffeine to liquid caffeineevaporation ; converts liquid caffeine to solidsublimation ; converts solid caffeine back to pure solid caffeineHence we can conclude that the states of change that solid caffeine passes through in order to become gas is ; Solid caffeine --- > liquid form -----> solid form ----->gaseous form .
Learn more : https://brainly.com/question/14446766
A gas has a volume of 550 mL at a temperature of -55 °C. The volume of the gas at 30 °C is
Blank 1:
mL.
Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
Which equation correctly converts degrees Celsius to kelvins?
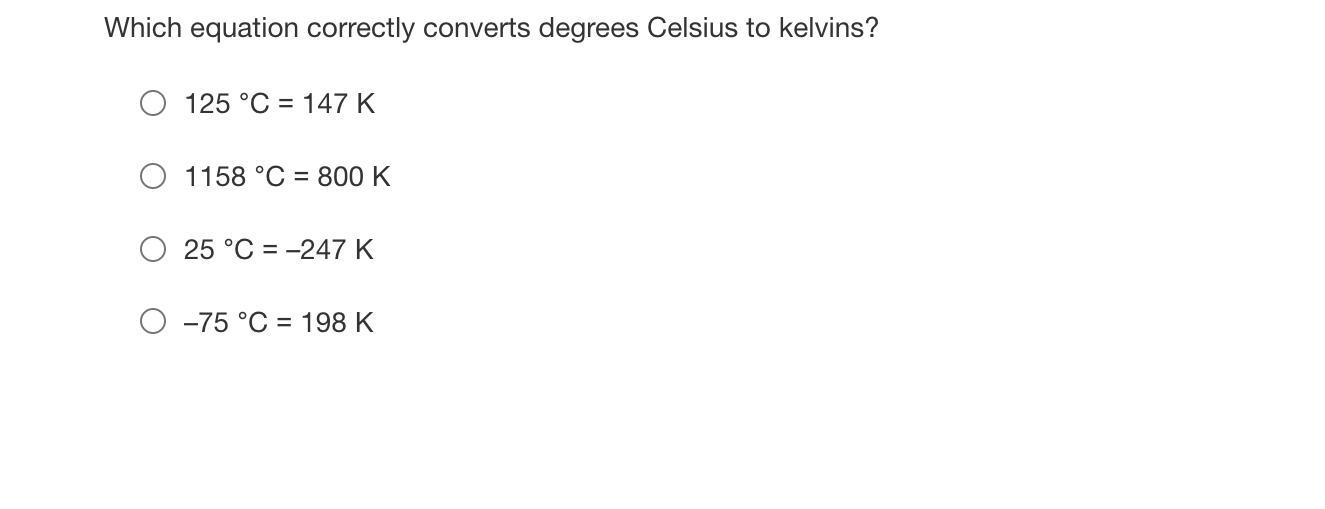
Answers
Answer:
D
Explanation:
To convert from celcius to kelvin add 273 to the celcius number. D has -75 celcius so if you add -75 to 273 you get 198 K
The correct equation that converts degree celsius to kelvin is -75°C = 198K
To convert from celcius to kelvin we have to add 273 to the celcius number.
i.e degree celsius + 273= kelvin
option D has -75 celcius so if you add -75 to 273 we get 198 K.
A kelvin is the same size as a Celsius degree.
Absolute zero is 0 K and the freezing point of water is 273.15 K.
To get the freezing point of water to be 0 °C, we must subtract 273.15 from the Kelvin reading (see Figure).
C = K – 273.15
C = 273.15 – 273.15
C = 0 °C
To get absolute zero on the Celsius scale, we repeat the process.
C = 0 – 273.15
C = –273.15 °C
To know more about kelvin here
https://brainly.com/question/11631512
#SPJ2
what is the periodic table elements
Answers
Answer:
Here is a picture of all the elements

Answer:
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
Explanation:
PLEASE MARK ME AS BRAINLIEST
If a person gains 72 m/s after covering 540m. Calculate the time taken.
Answers
Answer:
3.9 seconds
Explanation:
Given:
Final velocity = 72 m/sDistance covered = 540 mTo find:
Time taken = ?Solution:
We can use the equation,
v = u + at
where,
v is the final velocityu is the initial velocitya is the acceleration t is the time takenWe know that the initial velocity is 0, so the equation becomes,
v = at
We can also use the equation,
s = ut + ½* at²
where,
s is the distance coveredu is the initial velocity a is the acceleration t is the time takenWe know that the distance covered is 540 m and the final velocity is 72 m/s, so we can substitute these values into the equation to solve for the time taken.
540 = 0 * t + ½ * a * t²
540 = ½ * a * t²
1080 = a * t²
\(t^2= \frac{1080}{a}\)
\(t = \sqrt{ \frac{1080}{a}}\)
We know that the acceleration is the change in velocity divided by the time taken, so we can substitute this value into the equation to solve for the time taken.
\(t = \sqrt{\frac{1080 }{ 72 m/s}}\)
\(t = \sqrt{15} s\)
t = 3.9 s
Therefore, the time taken is t = 3.9 seconds
Please help!
Hydrochloric acid is a strong acid whereas acetic acid is a weak acid.
i. How would the pH of a 0.01M acetic acid compare to pH value for 0.01M HCl?
(Explain in your own words without calculating)
ii. Calculate the pH of a 0.01 M acetic acid.

Answers
Because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. 2.88 is the pH of a 0.01 M acetic acid.
What is acid?Any hydrogen that comprises a material capable of giving a proton (a hydrogen ion) to another chemical is defined as acid. A base is indeed a molecule or ion that can receive a hydronium ion from just an acid.
1)Because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. The pH value of stronger acid is lower.
2)CH\(_3\)COOH + H\(_2\)O ⇄ CH\(_3\)COO⁻+ H\(_3\)O⁺
0.01 0 0
-x +x +x
0.01-x +x +x
Ka=[ CH\(_3\)COO⁻][H\(_3\)O⁺]/[CH\(_3\)COOH]
1.8×10⁻⁵ = [x][x ]/[ 0.01-x ]
x=1.34×10⁻³
pH = -log[H⁺]
= -log[1.34×10⁻³]
=2.88
Therefore, because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. 2.88 is the pH of a 0.01 M acetic acid.
To learn more about acid, here:
https://brainly.com/question/29775793
#SPJ9
Lewis Structure for NO3-
Answers
Answer::
Explanation::
How many moles of Ne are contained in a 5.00 L tank at 155°C and 2.80 atm?
Answers
There will be 0.39 moles
Take a look at the He gas in the tank:
P = 2.80 atm, or pressure
Volume = 5.00 liters.
T = (273 + 155) is the temperature. K = 428 K
R = 0.08206 L atm/L of gas (mol K)
Charle's law, Boyle's law, and Gay-Lussac law are the three gas laws that make up the combined gas law, commonly referred to as a general gas equation. For a certain amount of gas, the law illustrates the relationship between temperature, volume, and pressure.
Gas law PV = nRT
So, n = PV/. (RT)
He gasses number = 2.80 x 5.00 / (0.08206 x 428) mol equals 0.39 mol.
Learn more about Gas law here-
https://brainly.com/question/12667831
#SPJ9