You started your preparation of common alum with 0. 9156 g of aluminum foil. What is your theoretical yield of alum?.
Answers
The theoretical yield of alum, calculated based on the given amount of aluminum foil (0.9156 g) and the stoichiometry of the reaction, is approximately 16.08 g.
Given that the formula of common alum is KAl(SO₄)₂.12H₂O and the starting amount of aluminum foil is 0.9156 g, the theoretical yield of alum can be calculated as follows:
1. Determine the number of moles of aluminum:
mol Al = mass of Al / molar mass of Al
mol Al = 0.9156 g / 26.98 g/mol
mol Al ≈ 0.0339 mol
2. Use the mole ratio between aluminum and alum from the balanced equation:
2 Al + K₂SO₄. Al(₂SO₄)3₃.24H₂O → 2 KAl(SO₄)₂.12H₂O + 3 H₂
The mole ratio indicates that 1 mole of aluminum reacts to produce 1 mole of alum.
3. Calculate the theoretical yield of alum:
mol alum = mol Al (from step 1) = 0.0339 mol
4. Convert the theoretical yield of alum to grams using its molar mass:
mass of KAl(SO₄)₂.12H₂O = mol alum x molar mass of KAl(SO₄)₂.12H₂O
mass of KAl(SO₄)₂.12H₂O = 0.0339 mol x 474.39 g/mol
mass of KAl(SO₄)₂.12H₂O ≈ 16.08 g
Therefore, the theoretical yield of alum is approximately 16.08 g.
Learn more about yield of alum here:
https://brainly.com/question/24123366
#SPJ11
Related Questions
Who is my favourite wrestler?
Answers
Answer:
ma herdina thesto wrestling
Question 5 (1 point)
Which statement is FALSE about VSEPR?
O a Shared electrons in bonds affect the shape of the molecule
Ob
VSEPR stands for Valence Shell Electron Pair Repulsion
Oc
When electrons are not shared equally, it causes the molecule to bend.
Od Lone pairs of electrons on the central atom affect the shape of a molecule.
Answers
The statement that is FALSE about VSEPR is "When electrons are not shared equally, it causes the molecule to bend"
Option C
What is VSEPR theory?VSEPR theory does not imply that unequal sharing of electrons causes a molecule to bend.
Rather, it is the repulsion between electron pairs, whether they are bonding or non-bonding, that determines the molecular geometry.
The VSEPR theory is based on the idea that valence electron pairs in the outermost shell of an atom repel each other, and the resulting repulsion determines the geometry of the molecule.
Therefore, Option C is false about VSEPR.
Learn more about VSEPR here: https://brainly.com/question/17177984
#SPJ1
How is carbon dioxide different than the other types of matter
Answers
How does the number of tin atoms in the sample compare to the number of aluminum atoms in a 20 gram sample of each (Sn:Al)
Answers
The number of Al atoms is greater than the number of Sn atoms
Further explanationGiven
20 gram sample of Tin and Aluminum
Required
Number of atoms
Solution
1 mol = 6.02 x 10²³ particles (atoms, molecules, ions)
So the number of atoms of each element is determined by the number of moles. The greater the number of moles the greater the number of atoms
moles Sn-Tin(Ar = 118,71 u)mol = mass : Ar
mol = 20 : 118,71 u
mol = 0.168
moles of Al-Aluminum(Ar=26.9815 u)mol = 20 : 26.9815 u
mol = 0.741
So the number of Al atoms > number of Sn atoms
3. What experimental factors are assumed to be constant in:
a. Pressure-Volume experiment?
b. Temperature-Pressure experiment?
Answers
In a Pressure-Volume experiment temperature and amount of gas is constant and In a Temperature-Pressure experiment volume and amount of gas is constant.
(a) In a Pressure-Volume experiment, the following factors are assumed to be constant:
1. Temperature: The experiment is typically conducted at a constant temperature. Any changes in pressure and volume are solely attributed to the manipulation of those variables, assuming temperature remains constant.
2. Amount of gas: The amount of gas in the system is usually kept constant throughout the experiment. This ensures that changes in pressure and volume are not influenced by changes in the number of gas molecules present.
(b) In a Temperature-Pressure experiment, the following factors are assumed to be constant:
1. Volume: The volume of the gas sample is typically kept constant during the experiment. This allows for the investigation of the relationship between temperature and pressure while eliminating the influence of volume changes.
2. Amount of gas: Similar to the Pressure-Volume experiment, the amount of gas in the system is generally kept constant. This ensures that changes in temperature and pressure are solely attributed to the manipulation of those variables, while holding the number of gas molecules constant.
By controlling these factors, researchers can isolate the effects of pressure and volume or temperature and pressure on gas behavior, allowing for a more precise understanding of the relationship between these variables.
Learn more about Pressure-Volume experiment here:
brainly.com/question/29516363
#SPJ11
can you help me with this screenshot thank you and I will choose you for brainist if you are the first 3 that answer
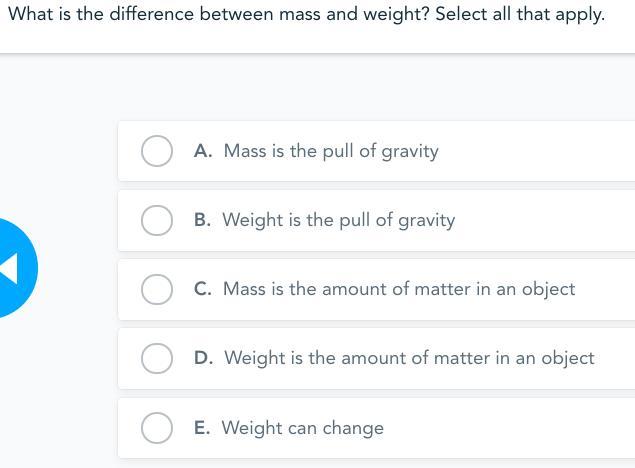
Answers
Your mass is the same no matter where you go in the universe your weight, on the other hand, changes from place to place. Mass is measured in kilograms even though we usually talk about weight in kilograms, strictly speaking it should be measured in newtons, the units of force.
Answer:
B. weight is the pull of gravity
C. mass is the amount of matter in an object
E. weight can change
How many molecules are in 0.400 moles of N2O5?
Answers
Answer:2.41 molecules
Explanation:
Answer:
2.41 molecules.
Explanation:
0.400 mol N2O5 contains 2.41 molecules N2O5.
1mole N2O5=6.022 × 1023molecules N2O5.
what systems might not work right when you have a cold
Answers
Answer:
hello :3
A cold is a contagious upper respiratory infection that affects your nose, throat, sinuses and trachea (windpipe).
Explanation:
have a nice day :3
when you divide the mass of a substance by its volume yu get ?
Answers
When you divide the mass of a substance by its volume you get its density.
What is density and its formula?Density is defined as mass per unit volume of a material or substance. The formula for density is d = M/V, where d represents density, M is mass, and V is volume. Density is commonly expressed in grams per cubic centimeter. Density depends on the mass of the body, higher the mass, higher will be the density.
So we can conclude that When you divide the mass of a substance by its volume you get its density.
Learn more about volume here: https://brainly.com/question/463363
#SPJ1
in part i, why do we use different concentrations for sulfuric acid and sodium hydroxide, 3 m h2so4 versus 6 m naoh?
Answers
Different concentrations of sulfuric acid and sodium hydroxide are used in Part I of a titration experiment to achieve the required stoichiometric ratio of 1:2, where a higher concentration of sodium hydroxide is needed to neutralize the acid.
Why different concentrations are used for sulfuric acid and sodium hydroxide?In Part I of a titration experiment, different concentrations are used for sulfuric acid and sodium hydroxide because their stoichiometric reaction requires different amounts of each reagent to neutralize the other. The reaction between sulfuric acid and sodium hydroxide involves a 1:2 ratio of acid to base, meaning that two moles of sodium hydroxide are required to neutralize one mole of sulfuric acid.
To achieve this stoichiometric ratio, a higher concentration of sodium hydroxide is needed relative to the sulfuric acid. Therefore, in the experiment, a 3 M solution of sulfuric acid is used while a 6 M solution of sodium hydroxide is used to ensure that the stoichiometric ratio is achieved when titrating the two solutions together.
Learn more about Titration experiment
brainly.com/question/16839748
#SPJ11
can you help me with this i don't think it is that hard i just have a lot on my mind.

Answers
Answer:
Global wind
Explanation:
The jet streams go around the whole earth from west to east
Hope you have a good day :)
select all the statements that correctly explain why ethylene has a zero dipole moment, whereas propene has a small dipole moment of 0.3 d.
Answers
b. Ethylene is symmetrical; all bond dipole moments cancel out.
In ethylene (C₂H₄), the molecule is symmetrical with a linear structure. Each carbon atom is bonded to two hydrogen atoms and shares a double bond with the other carbon atom. The electronegativity of carbon and hydrogen is similar, resulting in an equal sharing of electrons in the bonds. As a result, the bond dipole moments in ethylene are equal in magnitude but opposite in direction. The dipole moments cancel each other out, resulting in a net dipole moment of zero for the entire molecule.
In propene (C₃H₆), there is an additional methyl (CH₃) group attached to one of the carbon atoms. The methyl group has a slight electron-donating effect due to the presence of the carbon-hydrogen bonds. This creates a small imbalance in electron distribution within the molecule, resulting in a slight dipole moment. The dipole moment in propene is approximately 0.3 D, indicating a small separation of charges due to the presence of the methyl group.
Therefore, option b is correct as it explains that the zero dipole moment in ethylene is due to its symmetrical structure, while the small dipole moment in propene arises from the electron-donating ability of the methyl group.
To learn more about dipole moment, here
https://brainly.com/question/1538451
#SPJ4
The complete question is:
Select all the statements that correctly explain why ethylene has a zero dipole moment, whereas propene has a small dipole moment of 0.3 d.
a. small dipole moment in propene arises from electron-donating ability of methyl group
b. ethylene is symmetrical; all bond dipole moments cancel out
c. small dipole moment in propene arises from electron-withdrawing ability of methyl group
what is the amount of heat required to raise the temperature of 125.0 g of aluminum by 12c? (specific heat of aluminum
Answers
The amount of heat required to raise the temperature of 125.0 g of aluminum by 12°C is 1611 joules.
Temperature = 12°C
Mass = 125.0 g
To estimate the amount of heat required, we need to use the formula:
Q = m * c * ΔT
Q = the amount of heat in joules
m = the mass of the substance in kilograms
c = the specific heat capacity of the substance
ΔT = the change in temperature in degrees
The specific heat capacity of aluminum = 0.897 J/g°C.
Q = 125.0 g * 0.897 J/g°C * 12°C
Q = 1611 J
Therefore, we can conclude that the amount of heat required is 1611 J.
To learn more about the heat required
https://brainly.com/question/3727855
#SPJ4
the freezing point of water will be lowered most by dissolving 1.0 mole of group of answer choices naphthalene nacl mgcl2 ether
Answers
The freezing point of water will be lowered most by dissolving 1.0 mole of magnesium chloride (MgCl₂).
When a solute is dissolved in a solvent, such as water, it disrupts the orderly arrangement of water molecules, making it more difficult for them to form solid ice crystals. This disruption leads to a lowering of the freezing point of the solvent.
The extent to which the freezing point is lowered depends on the nature of the solute and its concentration. In this case, comparing the given options, dissolving 1.0 mole of magnesium chloride (MgCl2) will have the greatest effect on lowering the freezing point of water.
Magnesium chloride dissociates into three ions in water: one magnesium ion (Mg2+) and two chloride ions (Cl-). The presence of multiple ions increases the number of solute particles per mole, leading to a greater disruption of the water structure.
As a result, the freezing point depression caused by 1.0 mole of magnesium chloride is more significant compared to other solutes.
In contrast, naphthalene is a nonpolar solute and does not dissociate into ions in water. Sodium chloride (NaCl) dissociates into two ions, and ether is a nonpolar compound. Therefore, these substances would have a lesser effect on lowering the freezing point of water compared to magnesium chloride.
Learn more about freezing point at https://brainly.com/question/14738274
#SPJ11
Use the radius-ratio rule and the data in Table 15B.2 to predict the kind of crystal structure expected for (a) magne- sium sulfide, (b) lithium chloride, and (c) potassium bromide.
Answers
(a) Magnesium sulfide (MgS) is expected to have a tetrahedral crystal structure.
(b) Lithium chloride (LiCl) is expected to have an octahedral crystal structure.
(c) Potassium bromide (KBr) is expected to have a cubic crystal structure.
To predict the kind of crystal structure expected for magnesium sulfide (MgS), lithium chloride (LiCl), and potassium bromide (KBr) using the radius-ratio rule, we need to compare the ratio of the radii of the cation and anion with the critical values for different crystal structures.
The radius-ratio rule states that the ratio of the radii of the cation to the anion in an ionic compound determines the type of crystal structure it adopts.
We can find the critical radius ratio values for different crystal structures. Here are the values for some common crystal structures:
Coordination number 6 (octahedral): 0.414 - 0.732
Coordination number 8 (cubic): 0.732 - 1.0
Coordination number 4 (tetrahedral): 0.225 - 0.414
Now let's analyze each compound:
(a) Magnesium sulfide (MgS):
Mg²⁺ ion radius: 0.072 nm
S²⁻ ion radius: 0.184 nm
Radius ratio = (cation radius) / (anion radius) = 0.072 / 0.184 ≈ 0.391
Based on the radius ratio, which is less than 0.414, we can predict that magnesium sulfide (MgS) adopts a coordination number 4 (tetrahedral) crystal structure.
(b) Lithium chloride (LiCl):
Li⁺ ion radius: 0.076 nm
Cl⁻ ion radius: 0.181 nm
Radius ratio = (cation radius) / (anion radius) = 0.076 / 0.181 ≈ 0.420
The radius ratio for lithium chloride is between 0.414 and 0.732. This suggests that lithium chloride (LiCl) adopts a coordination number 6 (octahedral) crystal structure.
(c) Potassium bromide (KBr):
K⁺ ion radius: 0.138 nm
Br⁻ ion radius: 0.196 nm
Radius ratio = (cation radius) / (anion radius) = 0.138 / 0.196 ≈ 0.704
The radius ratio for potassium bromide is within the range of 0.732 - 1.0. This indicates that potassium bromide (KBr) adopts a coordination number 8 (cubic) crystal structure.
In summary:
(a) Magnesium sulfide (MgS) is expected to have a tetrahedral crystal structure.
(b) Lithium chloride (LiCl) is expected to have an octahedral crystal structure.
(c) Potassium bromide (KBr) is expected to have a cubic crystal structure.
Learn more about Critical Radius Ratio at
brainly.com/question/30655608
#SPJ4
kind of protists can live in groups with different individuals performing different jobs (site 1)

Answers
Answer:
The plasmodial slime molds are the kind of protist that can live in groups in which different individuals perform different jobs. Some kinds of bacterial cell walls also have other functions.
2Al + 3CuSO4–>3Cu+ Al2(SO4)3
If 2.75 g Al reacted completely with excess CuSO4 and the percent yield of Cu is 80.5%, what mass of Cu was produced?
Answers
Answer:
7.82 g of Cu
Explanation:
2 moles of Al react to 3 moles of copper sulfate in order to produce 3 moles of copper and 1 mol of aluminum sulfate.
Firstly we determine the moles of reactant.
As copper sulfate is in excess, Al is the limiting.
2.75 g . 1mol /26.98g = 0.102 moles
Ratio is 2:3. 2 moles of Al, can produce 3 moles of Cu
So the 0.102 moles of Al will produce(0.102 . 3) /2 = 0.153 moles.
We convert moles to mass: 0.153 mol . 63.5g /mol = 9.71 g
That's the theoretical yield (100 % yield reaction)
We know that: (yield produced / theoretical yield) . 100 = percent yield
We replace:
(Yield produced / 9.71g) . 100 = 80.5 %
(Yield produced / 9.71g) = 0.805
Yield produced = 0.805 . 9.71g = 7.82 g
What is the scientific notation of 65000?
Answers
Answer:
the answer is 6.5 x 10^4
Answer:
6.5 x 10^4
Explanation:
gold is one of the group Metals called the coinage metals. how many atoms of gold are in a u.s. eagle a gold alloy bullion coin with a mass of 31.1 g Au?
Answers
Answer:
9.51 x 10²² atoms Au
Explanation:(mass: 31.1g Au)
(m. mass: 196.967 g/mol Au)
(atoms: ?)
(31.1g Au x 1 mol Au x 6.022 x 10²³ atoms Au/ 196.967g Au x 1 mol Au)
Calculate the molar mass of aluminum oxide (Al2O3). Express your answer to four significant figures.
The molar mass of Al2O3 is
Answers
Answer:
102.0 g/mol.
Explanation:
Aluminum oxide or Al2O3 is a chemical compound composed of 3 Oxygen and 2 aluminum atoms. Naturally, it is present in the form of mineral corundum in crystalline polymorphic forms. It holds significant importance in the preparation of Aluminium metal that is used as a refractory material.
To calculate the molar mass of Aluminum oxide, we will add the molar mass of 2 Al and 3 O atoms.
Since molar mass of Al is: 26.98 g
Molar mass of O is: 16.00 g
Molar mass of Al2O3
= (26.98)2 + (16.00) 3
= 53.96 + 48
= 101.96 g/mol
Since it is mentioned in question to round off the answer upto 4 significant figures, it will make the answer: 102.0 g/mol.
I hope it helps!
Answer:
102 grams/mol
Explanation:
This is correct! :)
You see color when light waves are reflected off an object or ____ by an object
Answers
Answer:
absorbed
Explanation:
Different colors of light can be reflected or absorbed and that determines color. For instance an object that absorbs every color but green will appear green to the human eye.
(1) Ethane is chlorinated in a continuous reactor: Some of the product monochloroethane is further chlorinated in an undesired side reaction: (a) Suppose your objective is to maximize selectivity of the monochloroethane production relative to the dichloroethane production. Would you design the reactor for a high or low conversion of ethane? Explain your answer. What additional processing steps would almost certainly be necessary to make the process economical? (b) Draw and label a flowchart, assuming that the reactor feed contains only the two reactants. Use a degree-of-freedom analysis based on atomic species balances to determine how many process variable values must be specified for you to be able to calculate the remainder (c) The reactor is designed to yield a 15% conversion of ethane and a selectivity of 14 moles of C2HCl for every mole of C2H4C12. Assume all of the chlorine gas is reacted. Calculate the feed ratio (moles Cl2 /moles C2He) and the fractional yield of monochloroethane (d) Suppose the reactor is built and started up and the conversion is only 14%. Chromatographic analysis shows that there is no Cle in the product, but another species of molecular weight higher that that of dichloroethane is present.
Answers
In order to maximize the selectivity of the monochloroethane production relative to the dichloroethane production, the reactor must be designed for a low conversion of ethane.
What is selectivity? Selectivity is a term used to describe the extent to which a chemical reaction produces a single product rather than a mixture of multiple products. It is determined by dividing the amount of the desired product by the total amount of product(s) formed in the reaction. High selectivity implies that the desired product is produced in high quantities relative to undesired products.
A low conversion rate of ethane is preferable in this case, since the side reaction that creates dichloroethane is highly exothermic, thus raising the temperature and rate of this side reaction. So, in order to keep the production of the side product to a minimum, a lower conversion rate is needed. Processing Steps: Almost certainly additional processing steps would be required to make the process economical.
To know more about maximize visit:
https://brainly.com/question/30072001
#SPJ11
If 0.213 moles of argon occupies a volume of 652 mL at a particular temp and pressure, what volume would it occupy if 0.162 moles of argon were added at the same conditions?
Answers
Answer:
V₂ = 495.89 mL
Explanation:
Given data:
Initial number of moles = 0.213 mol
Initial volume = 652 mL
Final number of moles = 0.162 mol
Final volume = ?
Solution:
V₁/n₁ = V₂/n₂
By putting values,
652 mL/0.213 mol = V₂ /0.162 mol
V₂ = 652 mL 0.162 mol /0.213 mol
V₂ = 105.62 mL.mol /0.213 mol
V₂ = 495.89 mL
which part of the plant you think is the least important?
Answers
Answer:
Well maybe the pedals they don't help much
Explanation:
Answer:
brainliest please
Explanation:
the pollen probably because other plants have it
If you were presented with 2 liters of a 2m sucrose stock solution, how many grams of sugar would be in a 100ml aliquot?
Answers
Answer:
68.46 gms
Explanation:
2 molal solution means there is 2 moles of sucrose per kg of solvent (generally 1000 cc)
2 moles / 1000 ml * 100 ml = .2 moles
mole weight of sucrose = 342.3 gm/mole
342.3 gm/mole * .2 mole = 68.46 gms
A forest has both light colored trees and dark colored trees this same forest had both light colored months and dark colored months one year there is a disease that moves through the forest killing many of the light colored trees when the light colored trees were killed by a disease the forest became almost entirely composed of dark - colored trees generations later almost all months in the forest were dark in color instead of light which of the following gives the most likely explanation for why almost all the months were dark in color.
Answers
The dark moths were better hidden from predators and therefore more of them survived and this allowed them to have offspring.
Answer:
The dark moths were better hidden from predators and therefore more of them survived and this allowed them to have offspring.
Explanation:
If all of the light colored trees disappeared and there are only dark colored ones, then that means that the light colored moths wouldn't be able to live long since their traits wouldn't match the environment. This then leads to the conclusion that dark moths would be better hidden since their trait fits their current environment and would let them be hid much better from predators. This would allow them to survive longer and eventually, reproduce.
You are a paleontology professor working at a dig site looking for fossils. You come across a deposit that is emitting radiation. Upon further testing you find that the sample is changing from carbon (atomic number 6) into nitrogen (atomic number 7) as radiation is emitted. What type of radiation is it?
A. Gamma
B. Delta
C. Beta
D. Alpha
Answers
The type of radiation in this scenario is Beta and is denoted as option C.
What is Beta radiation?This is a high-speed electron which is emitted when the nucleus undergoes a radioactive decay during the process of beta decay.
In this scenario the electron is denoted as e with a superscript and subscript of 0 and -1 respectively which is the reason for the change from carbon to nitrogen.
Read more about Beta radiation here https://brainly.com/question/16645044
#SPJ1
write a net ionic equation to show why the solubility of co(oh)2(s) increases in the presence of a strong acid and calculate the equilibrium constant for the reaction of this sparingly soluble salt with acid.
Answers
The net ionic equation to show why the solubility of Co(OH)2(s) increases in the presence of a strong acid is as follows: Co(OH)2(s) + 4H+(aq) → Co2+(aq) + 4H2O(l).
In this reaction, Co(OH)2 is a sparingly soluble salt that undergoes dissolution in the presence of a strong acid. The acid, represented by 4H+, protonates the hydroxide ions (OH-) from Co(OH)2, forming water (H2O) and Co2+ ions. The presence of excess H+ ions shifts the equilibrium towards the formation of Co2+ ions, increasing the solubility of Co(OH)2. To calculate the equilibrium constant (K) for this reaction, we can use the expression: K = [Co2+]/[H+]^4, where [Co2+] represents the concentration of Co2+ ions and [H+] represents the concentration of H+ ions. The values of [Co2+] and [H+] can be determined experimentally, and plugging them into the equation will give the equilibrium constant (K) for the reaction.
Learn more about equilibrium constant here: brainly.com/question/29809185
#SPJ11
What is atomic size in periodic table?
Answers
Atomic size, also known as atomic radius, is a measurement of the size of an atom. It is one of the fundamental properties of elements in the periodic table and helps us understand the physical and chemical behavior of different elements.
Atomic size refers to the distance between the nucleus of an atom and the outermost electrons. It is usually measured in nanometers (nm) or picometers (pm).
The atomic size of an element can be determined by several factors, including the number of protons in the nucleus and the number of electron shells surrounding the nucleus. As a general rule, the atomic size decreases from left to right across a row in the periodic table and increases as you move down a column.
For example, the atomic size of lithium (Li) is 140 pm, while the atomic size of sodium (Na) is 186 pm. This is because sodium has one more electron shell than lithium, which makes it larger.
In addition to affecting the physical and chemical behavior of elements, atomic size also plays a vital role in determining the reactivity of different elements. For example, elements with smaller atomic sizes tend to be more reactive than elements with larger atomic sizes.
More facts about atomic size: https://brainly.com/question/21401913
#SPJ11
4. Consider the following statement made in Chapter 4 of the text: "... consider that if the
nucleus were the size of a grape, the electrons would be about one mile away on average."
Given that the average nucleus is 10-13 cm in diameter and the electrons move around the
nucleus at an average distance of 10-8 cm from it, is the above claim reasonably accurate?
Provide mathematical support.
Answers
The claim: "If the nucleus were the size of a grape, the electrons would be one mile away on average" is reasonably accurate because the ratios between the nucleus's sizes and the distances (between electrons and nucleus) for the two given examples are in the same order of magnitude.
To know if the claim is accurate we need to calculate the ratio of the size of the nucleus (the same as a grape) and the distance between the electrons and the nucleus for example 1 (r₁):
\( r_{1} = \frac{s_{1}}{d_{1}} \) (1)
and to compare it with the ratio of the size and the distance given in example 2 (r₂):
\( r_{2} = \frac{s_{2}}{d_{2}} \) (2)
Where:
s₁: is the size of the nucleus (like the size of a grape)
d₁: is the distance between electrons and nucleus of example 1 = 1 mile
s₂: is the average diameter of the nucleus = 10⁻¹³ cm
d₂: is the average distance between electrons and nucleus of example 2 = 10⁻⁸ cm
Assuming that the diameter of a grape is 3 cm (in a spherical way), the ratio of the first example is (eq 1):
\( r_{1} = \frac{3 cm}{1 mi*\frac{160934 cm}{1 mi}} = 1.86 \cdot 10^{-5} \)
Now, the ratio of the second example is (eq 2):
\( r_{2} = \frac{10^{-13} cm}{10^{-8} cm} = 1.00 \cdot 10^{-5} \)
Since r₁ and r₂ are in the same order of magnitude (10⁻⁵), we can conclude that the given claim is reasonably accurate.
You can learn more about the nucleus of an atom here: https://brainly.com/question/10658589?referrer=searchResults
I hope it helps you!