You probably use the term heat many times a day. As an example, when you cook, dinner, you heat your dinner on a hot stove. When you place a piece of cool metal in the sun, it is heated by the sun’s energy. Why is heat the correct term to use in these examples?
Answers
When you place a piece of cool metal in the sun, it is heated by the sun’s energy they show that heat flows from warmer to cooler
In the given example conduction concept are seen conduction means conduction is the transfer of heat energy from one substance to another or within a substance in which heat is transferred via conduction and when heat energy is converted through piece of metal then the substance itself does not flow and heat is transferred internally, by vibrations of atoms and molecules and electrons can also carry heat, which is the reason metals are generally very good conductors of heat
Know more about heat
https://brainly.com/question/28667134
#SPJ1
Related Questions
Suppose that 5.2 L of methane at a pressure
of 782 Torr is transferred to a vessel of volume
2.2 L. What is the final pressure of methane
if the change occurs at constant temperature?
Answer in units of Torr.
Answers
Answer:
Final pressure = 1848.36 Torr
Explanation:
Given that,
Initial volume, V₁ = 5.2 L
Initial pressure, P₁ = 782 Torr
Initial volume, V₂ = 2.2 L
We need to find the final pressure. We know that the relationship between pressure and volume is given by :
\(P\propto \dfrac{1}{V}\\\\\dfrac{P_1}{P_2}=\dfrac{V_2}{V_1}\\\\P_2=\dfrac{P_1V_1}{V_2}\\\\P_2=\dfrac{782\times 5.2}{2.2}\\\\P_2=1848.36\ torr\)
So, the final pressure is equal to 1848.36 Torr.
When 0. 4g of zinc trioxocarbonate (IV) reacted with dilute hydrochloric acid, zin chloride wa produced and carbon (IV) oxide evolved if the reaction took 2minute. What wa the rate of the reaction
Answers
The rate of reaction when 0. 4g of zinc trioxocarbonate (IV) reacted with dilute hydrochloric acid, zin chloride was produced and carbon (IV) oxide evolved if the reaction took 2minute is 0.0016 mol/min
The rate of reaction is the time taken for product molecules to appear or the time taken for reactant molecule to be converted and the rate of reaction = mole of product formed/time taken
And the equation of the reaction is
ZnCO₃ + 2HCl → ZnCl₂ + H₂O + CO₂
And the molar mass of zinc carbonate = 125.39 = 8g/mol
Mole of zinc carbonate converted = 0.4/125.38
Time taken = 2 min
Rate of reaction = 0.0032/2
Rate of reaction = 0.0016 mol/min
Know more about reaction
https://brainly.com/question/29346459
#SPJ4
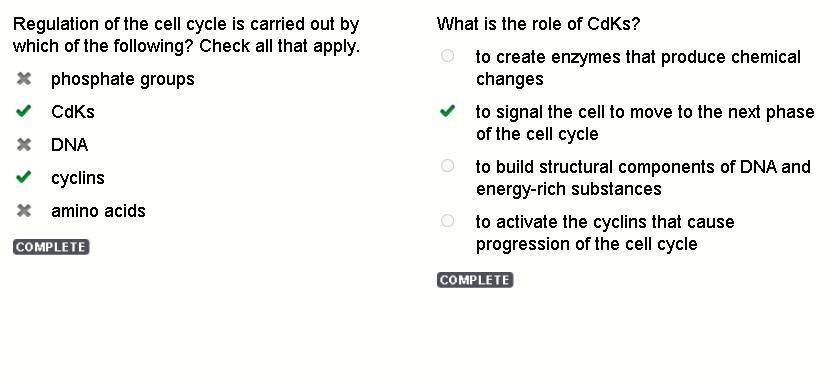
What is the role of CdKs? to create enzymes that produce chemical changes to signal the cell to move to the next phase of the cell cycle to build structural components of DNA and energy-rich substances to activate the cyclins that cause progression of the cell cycle
Answers
Answer:
It’s b
Explanation:
Answer:
Regulation of the cell cycle is carried out by which of the following? Check all that apply.
CdKs
cyclins
What is the role of CdKs?
to signal the cell to move to the next phase of the cell cycle
Explanation:
a chemist designs a galvanic cell that uses these two half-reactions: no3- 4h (aq) 3e
Answers
A chemist designs a galvanic cell by carefully selecting two compatible half-reactions—one for oxidation and the other for reduction. The half-reactions you provided represent the reduction half-reaction. The chemist would need to select an appropriate oxidation half-reaction to complete the design of the galvanic cell.
The half-reaction you mentioned is as follows:
NO3- + 4H+ + 3e- → NO(g) + 2H2O
In this reaction, nitrate ions (NO3-) and hydrogen ions (H+) combine with three electrons (3e-) to form nitrogen monoxide gas (NO) and water (H2O). This is the reduction half-reaction, as it involves the gain of electrons.
To construct a galvanic cell, two half-reactions are needed—one for the oxidation half-reaction and the other for the reduction half-reaction. The half-reaction you provided is the reduction half-reaction, which means it occurs at the cathode (the electrode where reduction takes place) in the galvanic cell.
For the oxidation half-reaction, the chemist would select a suitable reaction involving another species. The oxidation half-reaction will take place at the anode (the electrode where oxidation occurs). Without the information on the chosen oxidation half-reaction, I cannot provide a specific explanation. However, I can guide you through the general process.
To design the galvanic cell, the chemist must ensure that the reduction half-reaction and oxidation half-reaction are compatible. The oxidation half-reaction should involve a species that can provide the required number of electrons for the reduction half-reaction to occur.
Once both half-reactions are chosen, the next step is to assemble the galvanic cell. The two half-cells are connected by a salt bridge or a porous barrier to allow the flow of ions. The electrodes, where the half-reactions occur, are immersed in their respective electrolyte solutions.
During the operation of the galvanic cell, the oxidation half-reaction takes place at the anode, where oxidation occurs, and electrons are released. These electrons flow through an external circuit to the cathode, where reduction occurs. The reduction half-reaction consumes the electrons and produces the desired products.
As the oxidation and reduction half-reactions occur, ions flow through the salt bridge or porous barrier, maintaining charge balance and allowing the overall reaction to continue.
The galvanic cell produces an electric current that can be harnessed to power electronic devices or perform useful work. The magnitude of the electric current depends on factors such as the concentrations of the reactants, the nature of the electrode materials, and the overall cell potential.
Click the below link, to learn more about galvanic cell:
https://brainly.com/question/29784742
#SPJ11
* I NEED THIS *
Which of the following statements about oceans is incorrect?
A: Oceans collect water from other reservoirs including streams and rivers.
B: Oceans are the main source of drinking water on Earth's surface.
C: Oceans are connected to other reservoirs through evaporation and precipitation.
D: Oceans are the main type of reservoir that contain salt water.
Answers
Answer:
B is incorrect
Explanation:
Ocean are made of salt water which is undrinkable therefore they are not any type of drinking water source.
the latent heat of fusion for ice turning into water is 3.33 how many joules of heat to melt 5kg of ice initially at 0
Answers
The latent heat of fusion for ice turning into water is 3.33x105 J/kg. The number of joules of heat required to melt 5 kg of ice initially at 0°C is 1.665 x 106 J.
Given that,Mass of ice, m = 5 kg.
Latent heat of fusion for ice turning into water, L = 3.33 x 105 J/kg.
Heat energy required to melt the ice to water, Q = ?
Formula used:
Heat energy required to melt the ice to water,
Q = mL
The heat energy required to melt 5 kg of ice initially at 0°C can be determined using the formula,
Q = mL
Substituting the given values,
m = 5 kgL = 3.33 x 105 J/kg
Q = 5 kg × 3.33 x 105 J/kg
Q = 1.665 x 106 J
Therefore, the number of joules of heat required to melt 5 kg of ice initially at 0°C is 1.665 x 106 J.
To know more about latent heat of fusion visit:
https://brainly.com/question/87248
#SPJ11
Which of the following are typical of healthy water Water temperatures <86
∘
F for warm-water fisheries and <68
∘
F for cold-water fisheries Dissolved oxygen >5mg/L pH between 6 and 9 Streams should be "free from" sediments
Answers
These characteristics serve as guidelines for assessing the health and quality of aquatic environments, supporting the well-being of fish and other aquatic organisms.
Water temperatures below 86°F (30°C) for warm-water fisheries and below 68°F (20°C) for cold-water fisheries: Different fish species have different temperature preferences for optimal growth and survival. Warm-water fisheries generally thrive in higher water temperatures, while cold-water fisheries prefer cooler temperatures. Water temperatures outside these ranges can stress fish and disrupt their natural habitat.
Dissolved oxygen levels above 5 mg/L: Oxygen is essential for aquatic organisms, including fish, to breathe and carry out their metabolic processes. Adequate dissolved oxygen levels are necessary to support healthy aquatic ecosystems. Oxygen can enter the water through aeration from the atmosphere, photosynthesis by aquatic plants, and mixing with flowing water.
pH between 6 and 9: pH is a measure of the acidity or alkalinity of water. Most aquatic organisms, including fish, have adapted to function within a specific pH range. A pH between 6 and 9 is generally considered suitable for supporting diverse aquatic life. Extreme pH levels outside this range can be detrimental to aquatic organisms.
Streams should be "free from" sediments: Excessive sedimentation in streams can negatively impact aquatic ecosystems. Sediments can smother fish eggs, suffocate benthic organisms, and reduce water clarity, which affects photosynthesis and the availability of food sources. Healthy streams have a natural balance where sediment inputs are minimal, allowing for clear water conditions.
These characteristics serve as guidelines for assessing the health and quality of aquatic environments, supporting the well-being of fish and other aquatic organisms. However, it's important to note that specific water quality requirements may vary for different species and ecosystems.
Learn more about aquatic environments from below link
https://brainly.com/question/1023703
#SPJ11
Why does the battery give a reading of 9V even though there are no electrons flowing around the circuit?
Answers
Answer:
because battery have it's own voltage in it's composition
help me pleaseeeee!!!!!
Pure substances made of two or more kinds of atoms bound together
Compound
Mixture
Solution
Salt
Answers
a supramolecular sorting hat: stereocontrol in metal–ligand self assembly by complementary hydrogen bonding
Answers
The term "supramolecular sorting hat" refers to a concept in chemistry where metal-ligand self-assembly is controlled by complementary hydrogen bonding.
In this context, "supramolecular" means the assembly of molecules or ions into larger, more complex structures, and "sorting hat" is an analogy to the magical hat from Harry Potter that sorts students into different houses based on their characteristics. The "stereocontrol" in this process refers to the ability to control the spatial arrangement of the resulting supramolecular structures. This can be achieved by using ligands that have specific geometries and hydrogen bonding capabilities, which can selectively bind to metal ions and guide the assembly process.
The "complementary hydrogen bonding" refers to the formation of hydrogen bonds between the ligands and the metal ions. Hydrogen bonding is a type of intermolecular interaction where a hydrogen atom bonded to an electronegative atom (such as oxygen or nitrogen) is attracted to another electronegative atom. In summary, the term "supramolecular sorting hat: stereocontrol in metal-ligand self assembly by complementary hydrogen bonding" describes a process in chemistry where the self-assembly of metal-ligand complexes is controlled by complementary hydrogen bonding interactions, leading to the formation of specific supramolecular structures. This concept allows for the selective arrangement of molecules based on their geometries and hydrogen bonding capabilities.
To know more about supramolecular visit:
https://brainly.com/question/14495313
#SPJ11
1 mole of an ideal ga i expanded from a volume of 1. 00 liter to a volume of 8. 93 liter againt a contant external preure of 1. 00 atm. How much work (in joule) i performed on the urrounding?
Answers
The work (in joule) performed on the surrounding when 1 mole of an ideal gas expanded from a volume of 1. 00 liter to a volume of 8. 93 liter against a constant external pressure of 1. 00 atm is 803 joules
we know that,
n = 1 mole
Vi = 1 L
Vf = 8.93 L
P = 1 atm
the work performed on the surrounding under constant pressure is given as can be calculated as below:
W = P∆V
W = P(Vf—Vi)
W = 1 atm × (8.93—1) L
W = 1 atm × 7.93 L
W = 8.93 Litre•atm
Given that,
1 Litre•atm = 101.3J
So,
W = 7.93 Litre•atm × 101.3J/Litre•atm
W = 803.309 J
W ≈ 803 J
Then the work done on the surrounding is approximately
803 joules
Learn more about work here:
https://brainly.com/question/15688519
#SPJ4
How many grams of KCl are needed to make 50.0 mL of 2.45 M KCl a) 91.3 b) 9.13 c) 0.123 d) 1.52 e) none of the above
Answers
50.0 mL of 2.45 M KCl i.e potassium chloride require 9.13 grammes of KCl. The correct answer is option B.
To calculate the number of grams of KCl needed to make a solution, we can use the formula:
Mass (grams) = Volume (liters) × Concentration (Molarity) × Molar mass (grams/mol)
a) In this case, the volume is given as 50.0 mL, which is equivalent to 0.0500 liters. The concentration is given as 2.45 M, and the molar mass of KCl is 74.55 g/mol.
Mass (grams) = 0.0500 L × 2.45 M × 74.55 g/mol ≈ 9.11 grams
Therefore, the correct option is b) 9.13 grams.
To know more about potassium chloride, visit https://brainly.com/question/25380525
#SPJ11
ENT
Which of the following is one of Mendel's laws?
Law of Independent Assortment
ouTube
Law of DNA contains genes
Law of Segregation of Genes
Answers
Answer:
law of independent assortment
Some table salt substitutes have potassium chloride in them. If you consume potassium chloride, are you truly eating potassium and chlorine atoms? Support your answer with chemical evidence.
Answers
Answer:
You are consuming both
Explaination
2Na(s)+Cl two(g)
That gives us 2NaCl(s)
Suppose that in one year a country produces 2.92×10
11
kilowatt-hours (kWh) of electrical energy from 4088 hydroelectric plants (1.00kWh=3.60×10
6
J). On average, each plant is 90.0% efficient at converting mechanical energy to electrical energy, and the average dam height is 50.0 m. At 2.92×10
11
kWh of electrical energy produced in one year, what is the average power output P
avg
per P
avg
= hydroelectric plant? What total mass of water m flowed over the dams during that year? m= What was the average mass of water m
avg
per dam that provided the mechanical energy to generate the electricity? m
avg
= What was the average volume of water V
avg
per dam that provided the mechanical energy to generate the electricity? (The density of water is 1000 kg/m
3
.) V
avg
= m
3
A gallon of gasoline contains 4.50×10
7
J of energy. How many gallons n of gasoline did the 4088 dams save? n= galSuppose that in one year a country produces 2.92×10
11
kilowatt-hours (kWh) of electrical energy from 4088 hydroelectric plants (1.00kWh=3.60×10
6
J). On average, each plant is 90.0% efficient at converting mechanical energy to electrical energy, and the average dam height is 50.0 m. At 2.92×10
11
kWh of electrical energy produced in one year, what is the average power output P
avg
per P
avg
= hydroelectric plant? What total mass of water m flowed over the dams during that year? m= What was the average mass of water m
avg
per dam that provided the mechanical energy to generate the electricity? m
avg
= What was the average volume of water V
avg
per dam that provided the mechanical energy to generate the electricity? (The density of water is 1000 kg/m
3
.) V
avg
= m
3
A gallon of gasoline contains 4.50×10
7
J of energy. How many gallons n of gasoline did the 4088 dams save? n= gal
Answers
Part 1:Average power output per hydroelectric plant is 3.33 × 10^10 W. Part 2:Total mass of water flowed over the dams during that year is 2.41 × 10^13 kg. Part 3:Average mass of water per dam that provided the mechanical energy to generate electricity is 5.90 × 10^9 kg/dam. Part 4:Average volume of water per dam that provided the mechanical energy to generate electricity is 5.90 × 10^6 m³/dam.\ Part 5:Number of gallons of gasoline saved is 2.71 × 10^6 gallons.
Given data:
Total electrical energy generated by the hydroelectric plant = 2.92 × 10^11 kWh
Number of Hydroelectric Plants = 4088
Efficiency of each hydroelectric plant = 90% = 0.9
Dam height = 50.0 m
Density of water = 1000 kg/m³
Energy obtained per 1 kWh = 3.60 × 10^6 J
Conversion:1 kWh = 3.60 × 10^6 J
Part 1:Average power output per hydroelectric plant is given by;
Average Power = Total energy produced / TimeTotal Energy produced = 2.92 × 10^11 kWh × (3.60 × 10^6 J / 1 kWh)
Total Energy produced = 1.0512 × 10^18 J
Time = 365 days × 24 hours/day × 3600 seconds/hour
Time = 3.1536 × 10^7 s
Average Power = (1.0512 × 10^18 J) / (3.1536 × 10^7 s)
Average Power = 3.33 × 10^10 W
Part 2:
Total mass of water flowed over the dams during that year = (Energy produced / (Gravity × height of the dam × efficiency)) / Density
Energy produced = 2.92 × 10^11 kWh × (3.60 × 10^6 J / 1 kWh) = 1.0512 × 10^18 J
Density of water = 1000 kg/m³
Gravity = 9.8 m/s²
Height of the dam = 50.0 m
Efficiency = 0.9
Total mass of water flowed over the dams during that year = [(1.0512 × 10^18 J) / (9.8 m/s² × 50.0 m × 0.9)] / 1000 kg/m
³Total mass of water flowed over the dams during that year = 2.41 × 10^13 kg
Part 3:Average mass of water per dam that provided the mechanical energy to generate electricity = Total mass of water flowed over the dams / Number of hydroelectric plants
Average mass of water per dam = (2.41 × 10^13 kg) / 4088Average mass of water per dam = 5.90 × 10^9 kg/dam
Part 4:Average volume of water per dam that provided the mechanical energy to generate electricity = (Average mass of water per dam) / (Density of water)Average volume of water per dam = (5.90 × 10^9 kg/dam) / (1000 kg/m³)Average volume of water per dam = 5.90 × 10^6 m³/dam
Part 5:Energy contained in 1 gallon of gasoline = 4.50 × 10^7 J
Energy contained in the hydroelectric plant = (Energy contained in 1 gallon of gasoline) × (Number of gallons of gasoline saved)Energy contained in 1 gallon of gasoline = Energy produced / efficiency
Number of gallons of gasoline saved = (Energy produced / efficiency) / Energy contained in 1 gallon of gasoline
Number of gallons of gasoline saved = (2.92 × 10^11 kWh × 3.60 × 10^6 J / kWh) / (0.9 × 4.50 × 10^7 J)
Number of gallons of gasoline saved = 2.71 × 10^6 gallons
Therefore, the answer is
Part 1:Average power output per hydroelectric plant is 3.33 × 10^10 W.
Part 2:Total mass of water flowed over the dams during that year is 2.41 × 10^13 kg
.Part 3:Average mass of water per dam that provided the mechanical energy to generate electricity is 5.90 × 10^9 kg/dam.
Part 4:Average volume of water per dam that provided the mechanical energy to generate electricity is 5.90 × 10^6 m³/dam.
Part 5:Number of gallons of gasoline saved is 2.71 × 10^6 gallons.
To know more about hydroelectric plant , visit:
https://brainly.com/question/12021638
#SPJ11
How does the smell of the cooked food reach your nostrils without entering the kitchen?
Answers
Answer:
The smell of cooked food reaches our nostrils because particles of the aroma of food mix with the particles of the air and reach our nostrils through diffusion.
What is the molecular formula if the empirical formula is CH2O and the molecular molar mass is 180.18?
Answers
Answer:
The given chemical compound has 2 atoms of hydrogen and one atom of oxygen for each atom of carbon. The mass of CH2O is 12 + 2*1 + 16 = 30. The molecular weight of the compound is 180.18 which is approximately 180. This gives the molecular formula of the chemical compound as C6H12O6.
Next, break down the equation shown into the skeletal half-reactions for oxidation and reduction. Which of
these pairs shows the two skeletal half-reactions with their correct assignments?
reduction half reaction: HNO, NO
oxidation half reaction SH,SO,
oxidation half reaction: HNO3 -> NO
reduction half reaction: SH2SO,
reduction half reaction: HNO3 -H,SO,
oxidation half reaction S -> H2SO4
Answers
Answer:
Its answer A
Explanation:
I just took the test
Answer:
A:
reduction half reaction: HNO3-> NO
oxidation half reaction S->H2SO4
Explanation:
if u know that answer i will mark has brainliest

Answers
Answer:
The clear liquid is less dense than the black liquid, which is why it floats on top. If the volume were different, you would visually see more clear liquid than black and vice versa.
The clear liquid is less dense than the black liquid
Answer:
A. The clear liquid is less dense than the black liquid
Explanation:
Since the clear liquid since on top of the black one it's density is less. Whatever liquid has a higher density will sink to the bottom of the flask.
When NH3(aq) is added to an aqueous solution, a deep-blue color appears. What ion is present in the solution? a. Cu2+ b. Fe2+
c. Fe3+
d. d. Se
Answers
The ion that is likely present in the solution when a deep-blue color appears upon adding NH3(aq) is Cu2+.
When NH3(aq) is added to an aqueous solution, a deep-blue color usually appears due to the formation of a complex ion. The ion that is present in the solution depends on the metal ion present in the solution.
In the case of copper (Cu2+), adding NH3(aq) to an aqueous solution containing Cu2+ ions forms a deep-blue complex ion known as [Cu(NH3)4]2+.This complex ion is responsible for the observed blue color.
On the other hand, adding NH3(aq) to an aqueous solution containing Fe2+ ions forms a pale green complex ion known as [Fe(NH3)6]2+. This complex ion is not responsible for a deep-blue color.
Adding NH3(aq) to an aqueous solution containing Fe3+ ions forms a reddish-brown complex ion known as [Fe(NH3)6]3+. This complex ion is also not responsible for a deep-blue color.The ion Se is not known to form a deep-blue complex ion with NH3(aq).
To learn more about solution Click here:
brainly.com/question/30665317
#SPJ4
how long does it take for stirring and heat to dissolve in water?
Answers
Answer:
5-20 mins
Explanation:
Newtons’ THIRD Law of Motion
When 1 object exerts
a_____force_________ on a 2nd
object, the 2nd object exerts an
________and____________ force back on the 1st object.
Answers
the second one is equal and opposite
A sample of gas occupies a volume of 55.3 L, has a temperature of 23.3 °C and a pressure of .658 atm. Calculate the number of moles of gas which are present in the sample. R= .0821 atm L/mol K
Answers
Answer: The number of moles of gas which are present in the sample are 1.49 mol.
Explanation:
Given: Volume = 55.3 L
Temperature = \(23.3^{o}C\) = (23.3 + 273) K = 296.3 K
Pressure = 0.658 atm
Formula used is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\0.658 atm \times 55.3 L = n \times 0.0821 L atm/mol K \times 296.3 K\\n = \frac{0.658 atm \times 55.3 L}{0.0821 L atm/mol K \times 296.3 K}\\= \frac{36.3874}{24.32623}\\= 1.49 mol\)
Thus, we can conclude that the number of moles of gas which are present in the sample are 1.49 mol.
The kinetic energy of a moving object depends on its mass and its
a. volume.
b. velocity.
c. distance.
d. acceleration.
Answers
how many moles of fluorine atoms are contained in 3.0 moles of sulfur hexafluoride?
Answers
There are 18 moles of fluorine atoms in 3.0 moles of sulfur hexafluoride. Sulfur hexafluoride (SF6) is a covalent compound made up of one sulfur atom and six fluorine atoms.
To determine how many moles of fluorine atoms are contained in 3.0 moles of SF6, we need to first calculate the number of moles of SF6, and then multiply it by the number of fluorine atoms in one molecule of SF6. The molar mass of SF6 can be calculated by adding the atomic masses of one sulfur atom and six fluorine atoms. The atomic mass of sulfur is 32.06 g/mol, and the atomic mass of fluorine is 18.998 g/mol. Therefore, the molar mass of SF6 = (1 x 32.06 g/mol) + (6 x 18.998 g/mol) = 146.06 g/mol
Finally, we can calculate the number of moles of fluorine atoms in 3.0 moles of SF6 by multiplying the number of moles of SF6 by the number of fluorine atoms in one molecule of SF6. One molecule of SF6 contains six fluorine atoms, so Number of moles of fluorine atoms = 3.0 mol SF6 x 6 mol F / 1 mol SF6 = 18 mol F Therefore, there are 18 moles of fluorine atoms in 3.0 moles of sulfur hexafluoride.
To know more about moles visit ;
https://brainly.com/question/30885025
#SPJ11
What is the Gaseous product of photosynthesis?
Answers
Answer: oxygen
Explanations
The products of photosynthesis are glucose and oxygen.
what is the value for ∆soreaction for the following reaction, given the standard entropy values? 2h2s(g) so2(g) 3srhombic(s) 2h2o(g)
Answers
Given, the balanced chemical equation:
2H2S(g) + SO2(g) → 3S(rhombic) + 2H2O(g)
For which, the value of ∆So reaction is 177.4 J/mol.K.
Given, the balanced chemical equation:
2H2S(g) + SO2(g) → 3S(rhombic) + 2H2O(g)
We have to calculate the value of ∆So reaction using the standard entropy values.
∆So reaction = ΣSo products – ΣSo Reactants
The standard entropy values are:
ΔSo f(S) = 31.4 J/mol.K
ΔSo g(H2S) = 205.5 J/mol.K
ΔSo g(SO2) = 248.0 J/mol.K
ΔSo solid(rhombic) = 31.8 J/mol.K
ΔSo g(H2O) = 188.8 J/mol.K
ΔSo reaction = ΣSo products – ΣSo Reactants= [3 × ΔSo solid(rhombic) + 2 × ΔSo g(H2O)] – [2 × ΔSo g(H2S) + 1 × ΔSog(SO2)]= [3 × 31.8 + 2 × 188.8] – [2 × 205.5 + 1 × 248.0]= 177.4 J/mol.K
Therefore, the value of ∆Soreaction is 177.4 J/mol.K.
To know more about rhombic visit:
https://brainly.com/question/62039
#SPJ11
Sea, sand, sky, palms – made almost all of
Answers
Answer:
sup
Explanation:
which of the following is most likely to be found as a prosthetic group. (select all that apply) group of answer choices nadph glucose fad nad atp
Answers
A prosthetic group is a non-protein molecule that is tightly bound to a protein and is essential for its biological activity. Out of the given options, FAD and NAD are most likely to be found as prosthetic groups.
FAD, or flavin adenine dinucleotide, is a coenzyme that is involved in redox reactions, and is often tightly bound to enzymes involved in these reactions. Similarly, NAD, or nicotinamide adenine dinucleotide, is a coenzyme involved in redox reactions and is commonly found as a prosthetic group in enzymes such as dehydrogenases. Glucose and ATP are not likely to be found as prosthetic groups as they are not tightly bound to proteins, but rather serve as substrates or energy sources for various enzymatic reactions.
To know more about prosthetic visit:
https://brainly.com/question/31665776
#SPJ11
What will be the products in the reaction: AI + BaCI2
Answers
—->3Ba+2AlCl3 to balance the equation