Answers
Answer:
I think its 1.72 (:
Explanation:
Related Questions
A volume of 56.0 mL of He at STP. a. How much weight?
Answers
We already know that number of moles is 0.0025 mol.
So if we know the volume of the gas at STP and the number of moles we can get the mass of the gas.
V = 0.056 L
1 mol = 22.4 L
Molar mass of He = 4.0026 g/mol
So to calculate the weight we need number of moles and molar mass.
weight = 0.0025 mol x 4.0026 g/mol
= 0.01 g
Calculate the molarity of a solution consisting of 60.0 g of NaOH in 1.50 L of solution.
Answers
Explanation:
here's the answer to your question

Faculty of Science
Department of Chemistry
8/2019 SECOND SEMESTER EXAMINATIONS
351 STATISTICAL MECHANICS AND THERMODYNAMICS
INSTRUCTIONS: Answer any FOUR questions.
Pluck's constant, h: 6.26 x 1034 J.S
zmann constant, k: 1.38 x 10 JK
nic mass Unit, amu: 1.661 x 10-kg
Nag
3Higi
C₂ (N₂) = 27.9 +0.00418T JK mol
C₂ (H₂) = 29.6+0.00231T JK 'mol
C₂ (NH) 29.9+0.00261T JK 'mol"¹
Gas constant, R: 8.314 JK mol
Avogadro constant, L: 6.022 x 10 mol
Speed of light, C: 2.998 x 10" ms¹¹
1. (a) Deduce the translational, rotational and vibrational mode of motion for the following
molecules: (i) NHs (ii) CO₂ (iii) CH.
(b) Calculate the enthalpy change at 800 °C for the reaction:
2NH
اله
November, 2019
UNIT: 4
Time allowed: 3hrs
All 92.2 kJ/mol
=
2. (a) Sate the equipartition law of energy.
(b) Apply the classical theory in calculating the expected heat capacity at constant pressure
for H₂ and Cl2 molecule given that the experimental value for H₂ is 28.86 JK 'mol and that
of Cle is 34.51 JK 'mol Explain the variation in these values.
(b) What will be the C₂ for a monoatomic gas?
Answers
(a) (i) NH3: Translational, rotational, and vibrational modes of motion.
(ii) CO2: Translational and vibrational mode of motion.
(iii) CH4: Translational, rotational, and vibrational modes of motion.
What is a Vibrational Mode of Motion?This simply refers to the oscillatory movement of a physical system around its equilibrium position. This phenomenon is characterized by a complex interplay of forces and energy transfer mechanisms, which can lead to a wide range of intriguing dynamical behaviors.
The part b of the first question, the reaction given is incomplete, and the products are missing. Please provide the complete balanced chemical equation for the reaction so that the enthalpy change can be calculated.
Read more about motion here:
https://brainly.com/question/25951773
#SPJ1
2.00M CO and 2.00M H2O are mixed in a sealed container and the system is left to reach equilibrium. Use the following information to calculate the equilibrium constant, Kc if at equilibrium the concentration of CO2 is 0.73M
CO(g) + H2O(g) ⇌ CO2(g) + H2(g
Answers
The equilibrium constant, Kc, for the response is 0. This approach that at equilibrium, the attention of merchandise is correctly 0 in comparison to the attention of reactants.
The equilibrium constant, Kc, may be calculated the use of the concentrations of the reactants and merchandise at equilibrium, consistent with the regulation of mass action. The balanced chemical equation for the response is:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
At equilibrium, the attention of CO2 is given as 0.seventy three M. The preliminary attention of CO is 2.00 M, and the preliminary attention of H2O is likewise 2.00 M. Let x be the alternate in attention of CO and H2O, and y be the alternate in attention of CO2 and H2, then the equilibrium concentrations may be expressed as:
[CO] = 2.00 M - x
[H2O] = 2.00 M - x
[CO2] = 0.seventy three M + y
[H2] = y
The equilibrium expression for the response is:
Kc = ([CO2][H2])/([CO][H2O])
Substituting the equilibrium concentrations into the expression gives:
Kc = ([0.73 + y][y])/([(2.00 - x)][(2.00 - x)])
Assuming that x and y are small in comparison to the preliminary concentrations, we will approximate the equilibrium concentrations as:
[CO] ≈ 2.00 M
[H2O] ≈ 2.00 M
[CO2] ≈ 0.seventy three M
[H2] ≈ 0.00 M
Substituting those values into the equilibrium expression gives:
Kc = (0.730)/(2.002.00) = 0
This suggests that the response does now no longer continue to a enormous quantity withinside the ahead route and is in all likelihood to be an instance of a reversible response with a low equilibrium constant.
For such more questions on equilibrium
https://brainly.com/question/19340344
#SPJ11
Trial 1 shows a 1.691 gram sample of cobalt(II) chloride hexahydrate (MW = 237.93). What mass would we expect to remain if all the water is heated off?
Answers
The molar mass of cobalt (II) chloride hexahydrate is:
( 1 x molar mass of Co) + (2 x molar mass of Cl) + (6 x molar mass of H2O)
= (1 x 58.93) + (2 x 35.45) + (6 x 18.02)
= 237.93 g/mol
The mass of the water in the compound is:
(6 x molar mass of H2O)
= (6 x 18.02)
= 108.12 g/mol
So the mass of cobalt (II) chloride in the compound is:
1.691 g - 108.12 g/mol = 1.5829 g
If all the water is heated off, the remaining mass would be 1.5829 g.
What is meant by H, and 2H?
Answers
Explanation:
H is 1 mole of hydrogen
2H is 2 moles of hydrogen
An atom that has lost electrons is a:
Answers
Answer:
The atom that has lost an electron becomes a positively charged ion (called a cation)
PLEASE MARK ME BRAINLIESTAnswer: it’s a cation
Explanation:
The discovery that water vapor
will stick to smoke particles led
to the development of the
technology used to -
A treat sewage in settling tanks
B detect fires by measuring radiant
energy
C measure wind speeds produced by
tornadoes
D seed clouds to produce rain
Answers
The discovery that water vapor will stick to smoke particles is relevant to cloud seeding, which is the process of introducing substances into clouds to promote the formation of rain or other forms of precipitation.
By dispersing smoke particles or other suitable substances into clouds, water vapor can condense around these particles and form water droplets, eventually leading to the development of rain. This technology is used in situations where there is a need for additional rainfall, such as in areas experiencing drought or in agricultural settings where water is needed for crop growth. Cloud seeding can help enhance precipitation and increase water resources, contributing to water supply management and agricultural practices.
Hence, the correct statement is option d seed clouds to produce rain.
Learn more about cloud seeding here:
https://brainly.com/question/32119768
#SPJ 2
Write a K expression for
[CoCl4]2-(aq)(blue) + 6H2O(l) ⇌ [Co(H2O)6]2+(aq)(pink) + 4Cl-(aq)
Answers
The relationship between a reaction's products and reactants with regard to a certain unit is expressed by the equilibrium constant, K.
Thus, When a reaction is homogeneous, all of the products' and reactions' states of matter are the same (the prefix "homo" means "same"). The solvent typically controls the reaction's overall state of matter.
When a reaction is in chemical equilibrium, the concentrations of the reactants and products do not significantly fluctuate over time. A double arrow between the reactants and products denotes these reversible reactions.
As described, this reaction is endothermic, therefore when heat is added, the equilibrium constant moves to the right. In turn, this turns the solution blue. Cl- is drawn out of solution by the addition of AgNO3.
Thus, The relationship between a reaction's products and reactants with regard to a certain unit is expressed by the equilibrium constant, K.
Learn more about K expression, refer to the link:
https://brainly.com/question/4770891
#SPJ1
What is a solute? A. the substance that dissolves another substance.
Answers
When neutral, Ecorium has 126 protons and 126 electrons. Given that it has 6valence electrons, what is the effective nuclear charge felt by these outer electrons?
Answers
Answer:
\(+6\)Explanation:
Here, we want to calculate the effective neutral charge
From the question, there are 126 electrons
Since there are 6 valence electrons, the number of electrons residing outside the valence electrons is 120
The effective nuclear charge is the difference between the number of protons and the number of electrons outside the valence shell
Mathematically, we have it that:
\(Nuclear\text{ charge = 126 - 120 = +6}\)What are the characteristics of a gas?
A. no definite shape but a definite volume
B. a definite shape but no definite volume
C. a definite volume and definite shape
D. no definite shape or definite volume
Answers
Answer:
D. no definite shape or. definite volume
Explanation:
Gases do not have a definite shape or volume because the molecules in gases are very loosely packed, they have large intermolecular spaces and hence they move around. The particles of solid are closely packed and occupy less space while particles of gases are loosely packed and occupy the complete space available.
Hope this helps :)
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
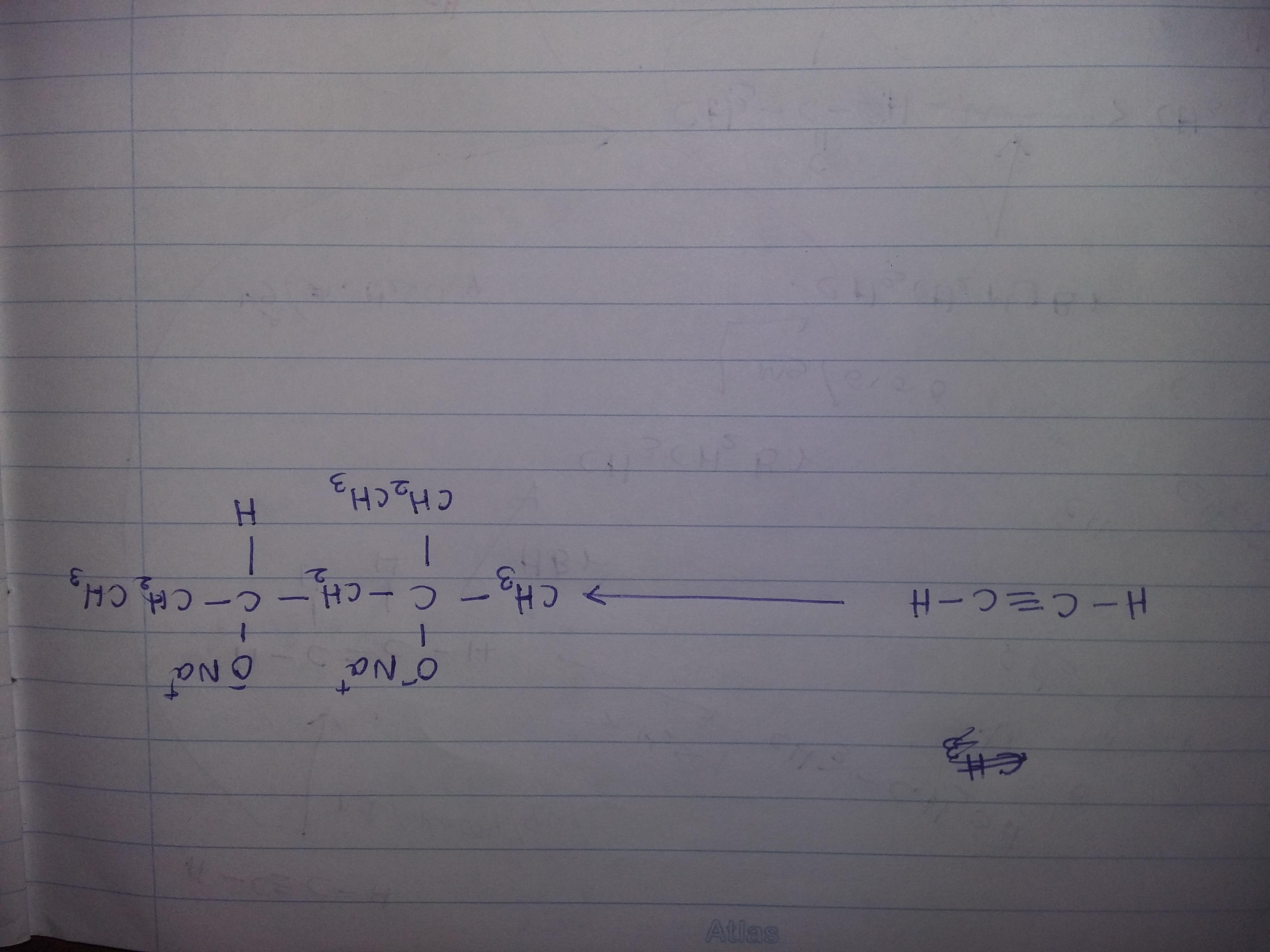
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
Which statement is true about a neutral solution? (5 points)
Its pH is less than 7.
Its pH is greater than 7.
It has the same concentration of hydronium and hydroxide ions.
It has a greater concentration of hydroxide ion than hydronium ions.
Answers
The statement "It has the same concentration of hydronium and hydroxide ions" is true about a neutral solution.
What is a neutral solution?If you've ever wondered what constitutes a neutral solution - it's one with an ideal pH balance set at precisely 7. Referred to as a 'neutral' because it doesn't lean towards acidity or alkalinity due to an equal concentration rate between hydronium ions (H+) and hydroxide ions (OH-).
Noteworthy examples can include distilled water, pure water, blood seawater, milk of magnesia among others.
Learn about neutral solution here https://brainly.com/question/21444245
#SPJ1
need help plz asap .......

Answers
Answer:
Purple flowers
Explanation:
Usually the dominant allele is a capital letter.
From the question, purple flowers are dominant to white flowers while white are recessive.
PP = Purple
pp = White
Answer:
Mostly purple flowers itself
what does GCAT help us remember?
Answers
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Which of the following is true about the elements on the Periodic Table? There may be 1 or more correct answers.
Answers
Answer: 1. Block
2. True ( I'm unsure. He arranged it according to mass, but he is credited for the periodic table)
3. Noble gasses
4. technetium
5. Alkaline earth metals
6. Number of protons
7. False
8. Not true: generally decreases as atomic number increases within a period
9. Argon
10. Four
11. False
12. False
Explanation:
Sodium hydroxide neutralises hydrochloric acid as shown in the equation:
NaOH(aq) + HCl(aq) i NaCl(aq) + H2O(l)
(4)
dm3
(3)
The student found that 27.20 cm3 of 0.100 moles per dm3 sodium hydroxide neutralised 5.00 cm3 of hydrochloric acid.
Calculate the concentration of the hydrochloric acid in moles per dm3.
Give your answer to three significant figures.
Answers
Explanation:
Mole ratio of NaOH : HCl = 1: 1
Moles of NaOH used = 0.1 mol/1000 cm3 × 27.20 cm3
= 2.72 × 10^-3 mol
Therefore moles of HCl used is also 2.72 × 10^-3 mol
So concentration of HCl can be found by dividing the no.of moles of HCl by the volume of HCl as follows
2.72 × 10^-3 mol/ 5cm3
1000cm3 = 1dm3
Therefore,
1cm3 = 1/1000 dm3
5cm3 = 5/1000 dm3
HCl conc. = 2.72 × 10^-3 mol/ 5×10^-3 dm3
= 0.544 moldm-3
Element Q has three isotopes. It is 18.75% -318, 78.26% Q-319, and 2.99% Q-320; The masses of the isotopes are 317.98 amu, 318.98 amu, and 320.026 amu respectively. What is the average atomic mass of the element Q
Answers
Answer:
Explanation:
ye
which element is most likely similar to oxygen in that it will form an ion with a 2- charge
Answers
Answer:
the answer is sulfur
Explanation:
Answer:
the answer is
Explanation:
sulfur
How do scientists test their ideas

Answers
1 points
A bottle contains a mixture of two gases: Oxygen and Hellum. The partial pressure of O2 is 1.0 atm and the partial pressure of He is 100.0 mmHg. What is the total pressure in the tank? (Volume and temperature are
constant)
101 alm
011 atm
O 101 mmHg
O 1.1 mmHg
Answers
101 mmHg is the total pressure in the tank.
Thus, Dalton's Law of Partial Pressures states that while the volume and temperature of a gaseous mixture are held constant, the total pressure of the mixture is equal to the sum of the partial pressures of its gaseous components.
Nitrogen, oxygen, argon, carbon dioxide, water vapor, and a trace amount of other gases are all present in atmospheric air and pressure.
The increased oxygen content in the chamber can displace the CO bound to hemoglobin faster than air oxygen, hence the hyperbaric chambers are also used to treat carbon monoxide (CO) poisoning. The treatment of scuba divers with the bends is another application for hyperbaric chambers.
Thus, 101 mmHg is the total pressure in the tank.
Learn more about Pressure, refer to the link:
https://brainly.com/question/30673967
#SPJ1
hi how do i do d (ii)? thanks!

Answers
A solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
We must first convert the amount of nitrate(V) ions in drinking water to moles per litre since the threshold concentration over which "Blue-Baby" Syndrome might manifest is 0.05 mg/cm3.
It is necessary to know the molar mass of nitrate(V) ions in order to convert from mg/cm3 to moles per litre (NO3-).
The formula below can be used to determine NO3-'s molar mass:
N: 14.01 g/mol for the atomic mass
O: 16.00 g/mol is the atomic mass (3 oxygen atoms in NO3-)
NO3-'s total molar mass is equal to 14.01 + 16.00 x 3 = 62.01 g/mol.
Let's now translate the specified concentration from mg/cm3 to moles/liter (M).
As 1 g = 1000 mg, 1 mg/cm3 is 0.001 g/cm3.
1 cm3 = 0.001 g/mL multiplied by 0.001 g/cm3
1 L/1000 mL x 0.001 g/mL = 0.001 g/L
We must change the grams into moles using the molar mass in order to get the molarity:
1.61 × 10-5 mol/L = 0.001 g/L / 62.01 g/mol
As a result, a solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
To know more about Blue-Baby" Syndrome:
https://brainly.com/question/31992990
#SPJ1
Using the law of the conservation of energy, types of energy transformations may occur in each
scenario below.
a. Logs burn in a fire
b. A waterfall turns the turbine to create electricity
c. A battery is used to power a flashlight
Answers
Types of energy transformations that may occur in each are heat, light, mechanical to electric, and chemical into heat and light energy.
a. Logs burn in a fire - chemical energy in the wood into heat and light energy.
b. A waterfall turns the turbine to create electricity - mechanical energy into hydroelectric energy.
c. A battery is used to power a flashlight - chemical energy into heat and light energy.
Even with the burning of timber, the chemical electricity within the wood is launched as warm due to the chemical response of wood and oxygen. This form of a chemical reaction is known as combustion and it calls for oxygen. Combustion converts the saved chemical strength inside the wood into heat and light electricity.
The regulation of conservation of power states that the amount of power is neither created nor destroyed. for example, while you roll a toy car down a ramp and it hits a wall, the power is transferred from kinetic power to capability electricity. Hydroelectric strength is additionally called hydroelectric electricity or hydroelectricity.
Learn more about energy transformations here:-https://brainly.com/question/2667612
#SPJ9
A 120 mL solution of sea water had 2.7x1022 sodium ions. What is the concentration of sodium in mol/L?
Answers
To calculate the concentration of sodium ions in sea water in units of mol/L, we need to use Avogadro's number to convert the number of ions to moles, and then divide by the volume of the solution in liters.
Avogadro's number is the number of atoms or molecules in one mole of a substance, and it is approximately 6.022 x 10²³.
First, we need to calculate the number of moles of sodium ions in the solution:
Number of moles of Na+ = (2.7 x 10²²) / (6.022 x 10²³) = 0.0448 mol
Next, we need to calculate the concentration of sodium ions in the solution:
Concentration of Na+ = (0.0448 mol) / (0.120 L) = 0.373 mol/L
Therefore, the concentration of sodium ions in sea water is approximately 0.373 mol/L.
To know more about Avogadro's number, visit:
https://brainly.com/question/11907018
#SPJ1
Easy Chemistry Questions!
1. What is the atomic symbol for silver?
2. What is the atomic mass of mercury?
3. Ni is the symbol for what element?
4. The element that has the atomic number 17 is?
5. List the symbols for two transition metals.
6. Cu, Ag, and Au are all in what group number?
7. Name two noble gases
8. Give the symbol for two halogens.
9. What is the symbol for element with atomic number 74?
10. What is the atomic mass of copper?
11. What is the last element in period 4?
Answers
Answer:
1. What is the atomic symbol for silver? Ag
2. What is the atomic mass of mercury? 200.59
3. Ni is the symbol for what element? Nickel
4. The element that has the atomic number 17 is? chlorine
5. List the symbols for two transition metals. Ir, Os
6. Cu, Ag, and Au are all in what group. # 11
7. Name two noble gases. helium, neon
8. Give the symbol for two halogens. F, Br
9. What is the symbol for element with atomic number 74? W
10. What is the atomic mass of copper? 63.55
11. What is the last element in period 4? krypton
Explanation:
in some applications nickel-cadmium batteries have been replaced by nickel-zinc batteries a single nickel-cadmium cell has a voltage of 1.30 V. Based on the idfference in the standard reduction potentials of CD2 and ZN2_, what votlage would you estimate a nickel-zinc a battery would produce
Answers
A mixture of 3 moles of N2, 5 moles of CO2, and 10moles of Cl2 exert a total pressure of 1120 mmHg. What is the partial pressure of CO2?
Answers
Answer:
A mixture of 3 moles of N2, 5 moles of CO2, and 10moles of Cl2 exert a total pressure of 1120 mmHg. What is the partial pressure of CO2?
Explanation:
According to Dalton's law of partial pressures:
The partial pressure of a gas can be determined by using the formula:
\(the partial pressure of a gas = mole fraction of the gas * total pressure\)
Partial pressure of CO2:
\(partial pressure of CO2= total pressure * mole fraction of CO2\\\\Mole fraction of CO2=\frac{number of moles of CO2}{total number of moles of all the gases} \\mole fraction of CO2=\frac{5mol}{3mol+5mol+10mol} = 5/18\\Partial pressure of CO2=\frac{5}{18} * 1120mmHg\\ =311.1mmHg\)
Hence, the partial pressure of CO2 is 311.1mmHg.
15. Excited sodium atoms emit light in the infrared at 589 nm. What is the energy of a single photon with this wavelength?
Answers
Answer:
3.36 × 10^-19 J
Explanation:
From the formula;
E= hc/wavelength
h= 6.6 ×10^-34 Js
c= 3 × 10^8 ms-1
Wavelength= 589 ×10^-9 m
E= hc/ wavelength
E=6.6 ×10^-34 × 3 × 10^-8/ 589 ×10^-9
E= 3.36 × 10^-19 J