Answers
Answer:111 meters
Explanation:
if you start at -86 meters and end at 45 an easy way is to take away the negative and add them together resulting in 111 (only works if you start or end negative and end the opposite.)
Related Questions
_Ag2S __Ag +_S8
I need help ASAP
Answers
Answer:
8Ag2S 16Ag + S8
Explanation:
What are the purpose of a chemical equation check all that apply
Answers
Answer: (A,B,D) [1, 2, 4]
Explanation:
How many particles would one formula unit of MgBr2 produce when dissolved in solution?
Answers
This question is asking for the number of particles that one formula unit of magnesium bromide will produce when dissolved in solution. At the end, the result turns out to be three (3) particles as shown below:
Ionization:
In chemistry, we understand ionization as a process whereby ionic substances split into ions as they dissolve in water. In this case, for magnesium bromide, MgBr₂, we see one Mg²⁺ and two Br⁻ ions are produced as shown below:
\(MgBr_2\rightarrow Mg^{2+}+2Br^-\)
Which means we will have three (3) particles when one formula unit of this salt dissolves.
Learn more about formula units: https://brainly.com/question/19293051
What is the formula for an ionic compound containing Ba²⁺ ions and Cl⁻ ions?
Answers
A compound consisting of Ba2+ and Cl- :
We have to cross multiply the ionic charges:
Therefore the compound will be:
\(\text{BaCl}_2\)
A student ran the experiment twice, using the same amount of copper both times. The first time he used water in the calorimeter. The second time he used automobile antifreeze, which has a specific heat of 2.41 J/g C. In which experiment did he obtain the higher final temperature in the calorimeter, with water or antifreeze? Explain your answer.
Answers
The student obtained a higher final temperature in the calorimeter when using water compared to using automobile antifreeze due to water's higher specific heat capacity (4.18 J/g°C).
To determine in which experiment the student obtained the higher final temperature in the calorimeter, we need to compare the heat capacities of water and automobile antifreeze.
Water has a specific heat capacity of 4.18 J/g°C, while automobile antifreeze has a specific heat capacity of 2.41 J/g°C. The specific heat capacity represents the amount of heat required to raise the temperature of a given substance by 1 degree Celsius per gram.
Since the student used the same amount of copper in both experiments, the heat transferred to the calorimeter (water or antifreeze) will depend on its heat capacity. A higher heat capacity means that more heat can be absorbed by the substance without a significant increase in temperature.
Since water has a higher specific heat capacity (4.18 J/g°C) compared to automobile antifreeze (2.41 J/g°C), it can absorb more heat per gram without a substantial rise in temperature. Therefore, when using water in the calorimeter, the student is likely to obtain a higher final temperature compared to when using antifreeze.
In summary, the student would obtain a higher final temperature in the calorimeter when using water rather than automobile antifreeze due to the higher specific heat capacity of water.
Know more about specific heat capacity here:
https://brainly.com/question/29792498
#SPJ8
List the two factors that affect ionic bond
Answers
Answer:
ionization energy and electronegativity
Explanation:
If you put 1.50 moles of N2 and 4.00 moles of O2 gas in a 45.0 L container at a temperature of 45°C, what is the total pressure of the system?
30 points
Answers
If you put 1.50 moles of N2 and 4.00 moles of O2 gas in a 45.0 L container at a temperature of 45°C, the total pressure of the system is 323.1 atm.
What ideal gas law ?The ideal gas law, also known as the general gas equation, is the state equation for a hypothetical ideal gas. Although it has several limitations, it is a good approximation of the behavior of many gases under many conditions.
Given;
N = 5.50 moles
V = 45.0 L
R = gas constant 8.314
T = 318 kelvin
P = ?
By an Ideal gas equation,
PV = nRT
By substituting this values in ideal gas equation, and we get as follows:
P = nRT / V
= 5.50 × 8.314 × 318 / 45.0
= 323.1 atm
Thus, the total pressure of the system is 323.1 atm.
To learn more about the ideal gas law follow the link;
https://brainly.com/question/13821925
#SPJ1
Nadia runs from her house to a fiend's house that is 24 meters away. How much time she will take to reach her friend's house, knowing that Nadia's speed is 3 m/s .
Answers
Nadia will take 8 seconds to reach her friend's house.
Speed is the measure of the distance traveled by an object per unit of time. It is a scalar quantity and is typically expressed in units such as meters per second (m/s), miles per hour (mph), or kilometers per hour (km/h).
To calculate the time Nadia will take to reach her friend's house, we can use the formula;
time = distance / speed
where distance is the amount of space traveled by an object, and time is the duration of travel.
Put the values given in the problem, we have:
time = 24 meters / 3 m/s
time = 8 seconds
Therefore, Nadia will take 8 seconds.
To know more about time here
https://brainly.com/question/15356513
#SPJ1
A student adds an alka-seltzer to the Koolaid and stirs. The pH meter now reads 8.3. What was released by the alka-seltzer tablet to cause this change?
Answers
Answer:
It released hydroxide ions (OH ¯).
Explanation:
A PH of 8.3 indicates that the solution has become a basic solution.
Now, for us to have a basic solution from the addition of an alka-seltzer to Koolaid, it means the Alka-seltzer released plenty of hydroxide ons (OH¯) to the solution.
HELPPPPP (100 POINTS)
Assume that the water stream is replaced by a stream of CCl4. Predict what would happen in each case.
a. charged acetate strip:
b. charged vinyl strip:
c. Explain your predictions.
Answers
Answer:c
Explanation:c
What determines the chemical properties and physical properties of a mineral?
the specific gravity of the mineral
о
the conditions under which the mineral formed
o the location where it was found
o
the arrangement of the mineral's atoms
Answers
Rubidium has a heat of vaporization of 69.0 kJ/mol at it's boiling point (686°C). Calculate ∆S for this process, Rb(l) -> Rb(g), at 1 atm and 685°C
Answers
From the calculation that we have here, the entropy of the system is obtained as 71.9 J/K.
What is the entropy?Entropy is a thermodynamic quantity that is a measure of the degree of randomness or disorder in a system. In other words, it is a measure of the number of possible ways that the energy of a system can be distributed, or the number of possible arrangements that the system can have.
We know that;
ΔS = ΔH/T
ΔS = 69 * 10^3/959
ΔS = 71.9 J/K
Learn more about entropy:https://brainly.com/question/13135498
#SPJ1
Some natural and synthetic materials are made of long repeating units. What are these best referred to as?
O Links (left) and chains (right)
O Molecules (left) and macromolecules (right)
O Solutes (left) and solutions (right)
O Monomer (left) and polymer (right)

Answers
Long repeating units are used to create several natural and manmade materials. they most appropriately referred to as O Links and chains (left) and O Molecules (right)? (left).
What is the name for a long sequence of repeated units?A molecule comprised of repeating units is referred to as a polymer. These "monomers" combine to form a lengthy molecular chain. It could be made up of branches or it might just be one continuous line of identically related molecules.
Name the worker macromolecule in the cell and describe one of its functions.A polymer or macromolecule made of many amino acids, proteins are. The monomers/building blocks of a protein are amino acids. things? There are numerous varieties of proteins, which are the supporting molecules of the cell,
To know more about macromolecules visit:-
https://brainly.com/question/6849865
#SPJ1
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
Of the following, ________ should have the highest critical temperature. Of the following, ________ should have the highest critical temperature. CH4 H2 CCl4 CBr4 CF4
Answers
Answer:
H2
Explanation:
Critical temperature is the temperature above which gas cannot be liquefied, regardless of the pressure applied.
Critical temperature directly depends on the force of attraction between atoms, it means stronger the force of higher will be the critical temperature. So, from the given options H2 should have the highest critical temperature because of high attractive forces due to H bonding.
Hence, the correct option is H2.
Answer:
CBr4
Explanation:
Critical temperature is dependent on the strength of the intermolecular forces.
First consider the types of intermolecular forces and the order of their strengths.
Dispersion forces < dipole-dipole forces < hydrogen bonding < ionic bonding
Remember, dispersion forces are present in all cases
H2: only dispersion forces are present
CH4, CCl4, CBr4, CF4: only dispersion forces are present
In order to break the tie we must start considering molar mass because larger molar masses correspond to larger intermolecular forces. Calculating molar mass shows that CBr4 is the largest and will have the strongest intermolecular forces and therefore will have the highest critical temperature.
The answer is CBr4
7.0 x 10 -3 mol of I2 in 100.00ml of solution
Answers
Given:
- Moles of I2: 7.0 x 10^(-3) mol
- Volume of solution: 100.00 mL (which is equal to 0.1000 L)
Molarity (M) = Moles of solute / Volume of solution in liters
Molarity = (7.0 x 10^(-3) mol) / (0.1000 L)
Molarity = 0.070 M
Therefore, the concentration of the I2 solution is 0.070 M.
Plants use sunlight to produce some ATP during photosynthesis. How do plants produce ATP when the Sun is not out?
Plants are secondary consumers.
Plants also use cellular respiration.
Plants extract ATP from the stars.
Plants are weak in the dark.
Answers
Answer:
The answer is
Plant also use cellular respiration
Answer:
B - Plants also use cellular respiration.
Explanation:
Did the test.
Why do elements not have a numerical value for standard heats of formation and Free energies of formation but do have a numerical value for standard molar entropies?
Answers
Because it takes no energy to generate a naturally occurring compound, the enthalpy of formation for an element in its elemental state will always be 0.
What do you mean by formation standard free energies?The free energy shift that happens when 1 mole of a material is created from its component elements in their standard states is referred to as the standard free energy of formation. The standard free energy of production of a pure element in its standard state is zero.
The distinction between Gibbs free energy and standard free energy is that the former is dependent on the experimental circumstances, whilst the latter describes the Gibbs free energy for reactants and products in their standard state.
learn more about standard free energy
https://brainly.com/question/14415025
#SPJ1
What type of cells are gametes?
Answers
Answer:
Reproductive cells(also known as sex cells) are gametes.
Explanation:
Have a great day :)
Answer:
Gametes are an organism's reproductive cells
Explanation:
What is the mass of 2.25 moles of sulfuric acid (H2 SO4)?
Answers
Answer:
The molar mass of sulfuric acid (H2SO4) can be calculated by adding the atomic masses of its constituent atoms:
Molar mass of H2SO4 = 2(1.008 g/mol) + 1(32.06 g/mol) + 4(15.99 g/mol) = 98.08 g/mol
Therefore, the mass of 2.25 moles of H2SO4 is:
mass = number of moles x molar mass
mass = 2.25 moles x 98.08 g/mol = 220.68 g
So, the mass of 2.25 moles of sulfuric acid is 220.68 grams.
What is used to determine the number of each atom in an ionic formula?
A. The oxidation state of each atom
B. The number of bonds in the formula
C. The electronegativity of each atom
D. The period of the metal in the formula
Answers
the oxidation state of each atom
Why is a hydrogen outer shell full with only two electrons, whilst a carbon outer shell needs eight electrons?
Answers
Hydrogen outer shell full with only two electrons, whilst a Carbon outer shell needs eight electrons to have completely filled electronic configuration.
Hydrogen has atomic number of 2 i.e., it has total of 2 electrons. As per the rule maximum of 2 electrons can be filled in first energy shell. In case of Hydrogen both the electrons are present in n = 1 shell, here n is principle quantum number. It has completely filled electronic configuration and also the most stable electronic configuration.
In case of Carbon, it has total of 6 electrons as it has atomic number 6. Out of 6 electrons two are filled in n = 1 shell , now we are left with 4 electrons. These four electrons are present in n = 2 shell. These four electrons are distributed as 2 electrons in 2s and 2 electrons in 2p and still we can fill 4 more electrons in 2p orbital to get completely filled electronic configuration which is also the stable one . So, Carbon outer shell needs eight electrons.
To know more about electronic configuration refer to the link:
https://brainly.com/question/26084288
#SPJ9
At a certain temperature it is found that 1.83 moles of H2, 2.33 moles of 02 and 3.95 moles of H2O are in equilibrium in a 8.1 L container according to the reaction below. What is the equilibrium constant?
2 H2 (g) + 02 (g) = 2 H20 (g)
Keep extra significant figures during the calculation and round your answer to 1 decimal place.
Answers
0.6 is the equilibrium constant for the given reaction.
To calculate the equilibrium constant (K) for the given reaction, we need to use the molar concentrations of the reactants and products at equilibrium. The equilibrium constant expression is given by:
\(K= [H_{2}O]^{2} / ([H_{2}^{2} * [O_{2}])\)
Given the moles of H2, O2, and H2O in the 8.1 L container, we can convert them to molar concentrations by dividing the number of moles by the volume:
[H2] = 1.83 moles / 8.1 L
[O2] = 2.33 moles / 8.1 L
[H2O] = 3.95 moles / 8.1 L
Substituting these values into the equilibrium constant expression, we have:
K = \((3.95 / 8.1)^{2}\) / (\((1.83 / 8.1)^{2}\) * (2.33 / 8.1))
Evaluating this expression and rounding to one decimal place, we find the equilibrium constant to be:
K ≈ 0.6
Therefore, the equilibrium constant for the given reaction is approximately 0.6.
Know more about equilibrium constant here:
https://brainly.com/question/3159758
#SPJ8
heck the box next to each molecule on the right that has the shape of the model molecule on the left:
Answers
Due to the core atom's (Br) sp3d2 hybridization, the molecule has an octahedral form, but Br has two lone pairs of electrons in the axial position, giving it a square planar shape.
All of the information on the chemical molecule bromine pentafluoride is provided in the hybridization of bromine pentafluoride notes. It is a hybrid molecule made up of five fluorine atoms, five pentavalent bromine atoms, and one pair of missing electrons. Since it is so reactive, oxidizers are added to chemical reactions and commercial rocket propellants.
A square pyramidal molecular structure is produced via the hybridization of bromine pentafluoride, which results in an sp3d2 hybridization. Due to the covalent electron sharing and a bond angle of roughly 90 degrees, the hybrid molecule features six sigma bonds. Two bromine electrons must move from its 4p orbital to its 4d orbital for hybridization to take place.
learn more about sp3d2 hybridization here:
https://brainly.com/question/30051973
#SPJ4
How much kinetic energy does a 6.08 kg ball have if it's moving with a speed of 1.14 m/s?
Answers
Answer: KE = 8.6917248 J
I'm not for sure but I am pretty positive this is the answer
The reaction in the diagram takes place in an ice calorimeter at 0°C.
What happens to the ice?
The temperature of the ice stays the same.
Some ice melts.
The ice gets colder.
You cannot tell from the information given.
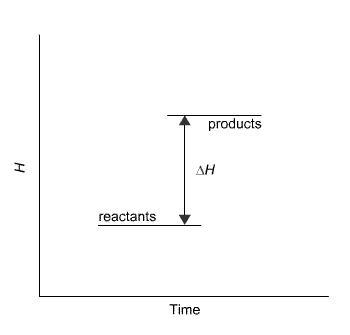
Answers
Which of the following pairs of solutions produces a precipitate when combined?
Cu(NO3)2 and NaCl
Cu(NO3)2 and K2CO3
CaCl2 and NaNO3
Fe(NO3)3 and MgCl2
Answers
The pairs of solutions that produces a precipitate when combined are \(Cu(NO_{3})_{2}\) and \(K_{2} CO_{3}\)3. So the correct option is B.
What is a precipitate?A precipitate is a solid that is created from a solution in an aqueous state in which two chemicals react. The precipitate then, once formed, will remain floating in solution. If you intervene in the process by putting the solution in a centrifuge, it will press the mass to the bottom of the tube, generating a compact mass.
The generation of the precipitate can be generated only if the characteristics of the compounds will overcome the solubility that it has to be able to generate a reaction.
All this will be generated because in the solution there will be components which will generate a chemical reaction between two ionic compounds. For this reason, the correct option will be B. \(Cu (NO_{3}) _{2}\) and \(K_{2} CO_{3}\).
To learn more about precipitate visit: https://brainly.com/question/29871773
#SPJ1
Find the mass of 3.9 x 1023 molecules of carbon dioxide gas at STP conditions. *
O 28.6 grams
O 67.7 grams
O 76.4 grams
O 19.1 grams
Answers
The molar mass (formula weight) of carbon dioxide (CO2) is 44.01 g/mol.
At STP (Standard Temperature and Pressure), which is defined as 0°C (273.15 K) and 1 atm of pressure, 1 mole of any gas occupies 22.4 L of volume.
Now, we have 3.9 x 10^23 molecules of CO2:
- To find the number of moles, we divide the number of molecules by Avogadro's number:
n = N/Na = 3.9 x 10^23/6.022 x 10^23 = 0.648 moles
- To find the mass, we multiply the number of moles by the molar mass:
mass = n x M = 0.648 mol x 44.01 g/mol = 28.52 grams
Therefore, the mass of 3.9 x 10^23 molecules of carbon dioxide gas at STP is approximately 28.6 grams (option A).
Suppose that you add 26.7 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f of 5.12 oC/m. With the added solute, you find that there is a freezing point depression of 2.74 oC compared to pure benzene. What is the molar mass of the unknown compound
Answers
Answer: The molar mass of the unknown compound is 200 g/mol
Explanation:
Depression in freezing point is given by:
\(\Delta T_f=i\times K_f\times m\)
\(\Delta T_f=2.74^0C\) = Depression in freezing point
i= vant hoff factor = 1 (for molecular compound)
\(K_f\) = freezing point constant = \(5.12^0C/m\)
m= molality
\(\Delta T_f=i\times K_f\times \frac{\text{mass of solute}}{\text{molar mass of solute}\times \text{weight of solvent in kg}}\)
Weight of solvent (benzene)= 0.250 kg
Molar mass of solute = M g/mol
Mass of solute = 26.7 g
\(2.74^0C=1\times 5.12\times \frac{26.7g}{Mg/mol\times 0.250kg}\)
\(M=200g/mol\)
Thus the molar mass of the unknown compound is 200 g/mol
The molar mass of an unknown solute compound in the solution has been 199.626 g/mol.
With the addition of the solute to the solution, there has been a depression in the freezing point. The depression in the freezing point can be expressed as:
Depression in freezing point = Van't Hoff factor × Freezing point constant × molality
The molality can be defined as the moles of solute per kg solvent
Molaity = \(\rm \dfrac{Mass\;of\;solute\;(g)}{Molecular\;mass\;of\;solute}\;\times\;\dfrac{1}{Mass\;of\;solvent\;(kg)}\)
The depression in freezing point can be given as:
Depression in freezing point = Van't Hoff factor × Freezing point constant × \(\rm \dfrac{Mass\;of\;solute\;(g)}{Molecular\;mass\;of\;solute}\;\times\;\dfrac{1}{Mass\;of\;solvent\;(kg)}\) ......(i)
Given, the depression in freezing point = 2.74 \(\rm ^\circ C\)
Van't Hoff factor = 1 (Molecular compound)
Freezing point constant (Kf) = 5.12 \(\rm ^\circ C\)/m
Mass of solute = 26.7 g
Mass of solvent = 0.250 kg
Substituting the values in equation (i):
2.74 \(\rm ^\circ C\) = 1 × 5.12
\(\rm \dfrac{2.74}{5.12}\) = \(\rm \dfrac{1}{Molecular\;mass\;of\;solute}\;\times\;\dfrac{26.7}{0.250\;kg}\)
0.535 = \(\rm \dfrac{1}{Molecular\;mass\;of\;solute}\;\times\;106.8\)
Molecular mass of solute = \(\rm \dfrac{106.8}{0.535}\) g/mol
Molecular mass of solute = 199.626 g/mol
The molar mass of an unknown solute compound in the solution has been 199.626 g/mol.
For more information about the molality of the compound, refer to the link:
https://brainly.com/question/7229194
Which of the following contains the atom with the highest oxidation number?
a. NaClO4
b. FeCl3
c. H2O2
d. SnH4
e. CrO3
Answers
The compound that contains the atom with the highest oxidation number is CrO₃ (option E).
What is oxidation number?Oxidation number is the hypothetical charge of an atom within a molecule or compound.
The oxidation number of an atom or ion determines the subscript given to the other elements in the molecule.
According to this question;
in the molecule: CrO₃, chromium has an oxidation number of +6 while oxygen has an oxidation number of -2. in the molecule: FeCl₃, iron has an oxidation number of +3 while chlorine has an oxidation number of -1.Therefore, it can be said that the molecule that posseses the atom with the highest oxidation number is CrO₃.
Learn more about oxidation number at: https://brainly.com/question/15167411
#SPJ1