Answers
Answer:
The concentration of the acid, HCl is 0.013 M
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
2HCl + Ca(OH)₂ —> CaCl₂ + 2H₂O
From the balanced equation above,
The mole ratio of the acid, HCl (nₐ) = 2
The mole ratio of base, Ca(OH)₂ (n₆) = 1
Finally, we shall determine the molarity of the HCl. This can be obtained as follow:
Volume of acid, HCl (Vₐ) = 250 mL
Molarity of base, Ca(OH)₂ (M₆) = 0.118 M
Volume of base, Ca(OH)₂ (V₆) = 13.7 mL
Molarity of acid, HCl (Mₐ) =?
MₐVₐ / M₆V₆ = nₐ/n₆
Mₐ × 250 / 0.118 × 13.7 = 2/1
Mₐ × 250 / 1.6166 = 2
Cross multiply
Mₐ × 250 = 1.6166 × 2
Mₐ × 250 = 3.2332
Divide both side by side 250
Mₐ = 3.2332 / 250
Mₐ = 0.013 M
Thus, the concentration of the acid, HCl is 0.013 M
Related Questions
represents the covalent bond by dashes and shows the arrangement of covalently bonded atoms _____.
Answers
Answer:
13
Explanation:
Atoms are thermally promoted to the excited state through collisions in the plasma. As they return to a lower energy state, they emit photons.
a. True
b. False
Answers
Answer:
True
Explanation:
1. If you place 30.0 L of ethyl acetate (C4H8O2) in a sealed room that is 7.25 m long, 2.75 m wide, and 2.75 m high, will all the ethyl acetate evaporate? If some liquid remains, how much will there be? The vapor pressure of ethyl acetate is 94.9 torr at 25 °C, and the density of the liquid at this temperature is 0.901 g/mL. Treat the room dimensions as exact numbers.
Answers
There will be 0.4589 mL of ethyl acetate left in the space after evaporation.
What is evaporation?The conversion of a liquid substance into a gas is known as evaporation. As a result of the liquid absorbing energy from its surroundings, molecules begin to travel faster and faster until they finally become a vapour and escape into the environment. Usually, the energy is absorbed as heat, but it can also be in the form of light or electricity.
No, the ethyl acetate won't all evaporate. The amount of ethyl acetate that will stay in the space after evaporation can be determined using the ideal gas law. As per the ideal gas law, PV = nRT
P is the overall system pressure, V is the room's volume, n is the amount of ethyl acetate in moles, R is the ideal gas constant, and T is the temperature.
To solve for n, the quantity of moles of ethyl acetate, we can rearrange the equation as follows: n = PV/RT
When the values are plugged in, we get:
n = (94.9 torr)(7.25 m x 2.75 m x 2.75 m)/(8.314 J/K mol)(298 K)
\(n = 4.666 \times 10^{-3} mol\)
The molar mass of ethyl acetate (88.11 g/mol) can then be used to compute the mass of ethyl acetate:
Mass = \(n \times M = (4.666 x 10^{-3} mol)(88.11 g/mol)\) = 0.4125 g
Using the density of ethyl acetate (0.901 g/mL), it is possible to determine the volume of the liquid that is still present:
Volume = mass/density = (0.4125 g)/(0.901 g/mL) = 0.4589 mL
As a result, there will be 0.4589 mL of ethyl acetate left in the space after evaporation.
To learn more about evaporation, visit:
brainly.com/question/24258
#SPJ1
Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. A precipitate forms when aqueous solutions
of lead(II) nitrate and chromium(II) sulfate are combined. Do not include states such as (s) or (aq)It is not necessary for you to indicate which of the products is the precipitate
Answers
The balanced chemical equation for the double-replacement precipitation reaction between lead(II) nitrate and chromium(II) sulfate can be written as: Pb(NO3)2 + CrSO4 → PbSO4 + Cr(NO3)2
What is the balanced chemical equation for the double-replacement precipitation?In this reaction, lead(II) nitrate and chromium(II) sulfate react to form lead(II) sulfate and chromium(II) nitrate. The lead sulfate is insoluble in water and precipitates out of the solution, while the chromium nitrate remains in the solution as a soluble salt.
Thus, the balanced chemical equation for the double-replacement precipitation reaction between lead(II) nitrate and chromium(II) sulfate can be written as:
Pb(NO3)2 + CrSO4 → PbSO4 + Cr(NO3)2
Learn more about double displacement here: https://brainly.com/question/26413416
#SPJ1
Aluminium+Sulphuric acid =Aluminium Sulphate + hydrogen
Answers
Answer:
2Al+3H2SO4 ---> Al2(SO4)3+3H2
Explanation:
same amount of al, h, and so4 on each side
1000 in² = ? yd.
Could sb help me out
Answers
Answer:
um 27 yards..............
The correct number of significant figures in the number 9.080 x10^4 is:
Answers
The correct number of significant figures in the number \(9.080 \times 10^4\) is 4.
What are significant figures?Significant figures refer to the digits in a number that are trustworthy and denote the amount of something, also known as the significant digits, accuracy, or resolution.
Only the digits allowed by the measurement resolution are dependable, hence only these can be important figures if a number expressing the outcome of a measurement (such as length, pressure, volume, or mass) has more digits than the number of digits allowed by the measurement resolution.
Some rules to understand significant figures:
1. Non-zero digits are always significant
2. Zeros between non-zero digits are always significant.
3. Leading zeros are never significant.
4. Trailing zeros are only significant if the number contains a decimal point
This number has 4 significant figures.
To know more about significant figures, click the link given below:
brainly.com/question/29153641
#SPJ1
certain experiment, 0.969 mol sample of Cu is allowed to react with 246 mL of 6.60 M HNO3 according to the following action: મા
Cu(s) + HNO3(aq) → Cu(NO3)2(aq) + H2O(l) + NO(g)
Istan
a) What is the limiting reactant?
b) How many grams of H2O is formed?
c) How many grams of the excess reactant remain after the limiting reactant is completely consumed?
Answers
Copper is the limiting reactant, with n(Copper) = 0.969 mol being less than n(Nitric acid). It produces 17.44 grammes of water. As the outcome is negative, there is no excess Nitric acid. The reaction uses up all of the Nitric acid.
How are charges balanced in a redox reaction?The method described in the following steps can balance a redox equation: (1) Split the equation into two equal halves. (2) Make each half-mass reaction's and charge equal. (3) Ensure that the quantity of electrons going to each half-reaction is the same. The half-reactions should be combined.
n(Nitric acid) = (246 mL) x (6.60 mol/L) = 1.6236 mol
Since n(Copper) = 0.969 mol is less than n(Nitric acid), Copper is the limiting reactant.
m(Water) = n(Water) x M(Water) = 0.969 mol x 18.015 g/mol = 17.44 g
Therefore, 17.44 grams of Water is formed.
n(Nitric acid) needed = n(Copper) x 2 = 1.938 mol
The excess amount of Nitric acid is:
n(Nitric acid) excess = n(Nitric acid) initial - n(Nitric acid) needed
= 1.6236 mol - 1.938 mol
= -0.3144 mol
To know more about limiting reactant visit:-
https://brainly.com/question/14225536
#SPJ1
Electrons are embedded in a mass of positively
charged matter.
Answers
Answer:
plum pudding model .
Explanation:
the electrons were 'like plums embedded in a pudding'. Also called the Raisin Bread Model.
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
Why do all elements have their own unique color? Please explain.
Answers
All the element have their own unique color because the every element have their own set of energy levels.
According to the bohr model of atoms : electrons exist at certain energy levels. when we give heat to electron , it gets excited and moves from lower energy level to higher energy level. the electron is less stable in higher energy level. when an electron returns from higher energy level to lower energy level it emits some energy in form of radiation. The wavelength of light depends upon energy level . and every elements have their own unique energy levels. The color for different element is different.
Thus, All the element have their own unique color because the every element have their own set of energy levels.
To learn more about element colors here
https://brainly.com/question/5809727
#SPJ1
Hydrogen gas reacts with oxygen gas to produce water at STP.
How many liters of hydrogen gas are required to produce 25.0 grams of
water?
Answers
The volume of hydrogen gas required to produce 25.0 grams of water at STP is 31.2 liters.
What is the volume of hydrogen gas produced?The balanced chemical equation for the reaction between hydrogen gas and oxygen gas to produce water is:
2H2(g) + O2(g) → 2H2O(l)
From the equation, we can see that 2 moles of hydrogen gas are required to produce 2 moles of water. The molar mass of water is 18 g/mol, so 2 moles of water has a mass of 36 g.
Therefore, to produce 25.0 grams of water, we need to use:
(25.0 g / 36.0 g/mol) * 2 mol H2/mol H2O = 1.39 mol of H2
At STP (standard temperature and pressure), 1 mole of gas occupies 22.4 L of volume.
So, 1.39 moles of H2 will occupy:
1.39 mol x 22.4 L/mol = 31.2 L
Learn more about volume of hydrogen gas here: https://brainly.com/question/17169574
#SPJ1
If a gas sample has a pressure of 74 ka at 87 L, what would the new volume be if the pressure changed to 929 kPa?
Answers
Answer:
La ley de los gases ideales relaciona cuatro propiedades macroscópicas de los gases ideales (presión, volumen, número de moles y temperatura). Si conocemos los valores de tres de estas propiedades, podemos utilizar la ley de los gases ideales para conocer la cuarta. En este video, usaremos la ley de los gases ideales para resolver el número de moles (y en última instancia de moléculas) en una muestra de un gas
Explanation:
34.1 mg NaCl in 117.3 mL of solution
Answers
The molarity of the solution will be 4.97 M.
What is molarity?It is the number of moles of solutes per 1 liter of solvent or solution.
Mole of 34.1 g NaCl = 34.1/58.44 = 0.5835 mol
117.3 mL = 117.3/1000 = 0.1173 L
Molarity of the solution = 0.5835/0.1173 = 4.97 M
More on molarity can be found here: https://brainly.com/question/2817451
#SPJ1
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
When three candles are placed under the test tube, the water level will rise higher. Which ofthe following statements would account for this difference? O Heat reduces pressure inside the test tube.O There is a directly proportional relationship between the amount of heat and the amount ofwater pulled into the test tube.O Three candles generate more heat.O Three candles use more oxygen creating a large vacuum.
Answers
Answer:
Explanation:
Here, we want to get the factor that is responsible for the observation
According to Charles' law, the volume of a given mass of gas is directly proportional to the temperature
What this means is that as the heat is increased, the volume is increased too
When the
how does ease of ion pair formation depend on concentration.
Answers
The median of the following set of data (39.8, 39.6, 39.2, 39.6 and 39.5) is:
Answers
The median of the following set of data (39.8, 39.6, 39.2, 39.6 and 39.5) is 39.6.
What is median?Median is a number separating the higher half from the lower half of a data sample, population, or probability distribution.
The median of a finite list of numbers can be found by arranging all the observations from lowest value to highest value and picking the middle one (e.g., the median of {3, 3, 5, 9, 11} is 5.
The following set of data was given this question;
39.8, 39.6, 39.2, 39.6 and 39.5
We arrange these data as follows:
39.2, 39.5, 39.6, 39.6, 39.8
The middle number or median is 39.6
Learn more about median at: https://brainly.com/question/28060453
#SPJ1
(12 points total) a researcher prepares a 1.00 m (molal) solution of acetone (ch3coch3) dissolved in ethanol (c2h5oh). given the densities of acetone and ethanol are 0.788 g/ml and 0.789 g/ml, respectively, calculate the concentration values of acetone below. assume that the final volume equals the sum of the volumes of acetone and ethanol. remember to provide units.
Answers
Acetone has a concentration of 0.74 Mm.
What is acetone?Acetone is an organic chemical that is colorless, combustible, volatile, and has a distinctively strong odor. It is a solvent that is frequently used in a variety of commercial and domestic settings. It also serves as a starting point for the synthesis of other chemicals. Acetone has the chemical formula CH3COCH3 and a molecular weight of 58.08 g/mol.
How do you determine it?We must first determine how many moles of acetone are present in the solution.
Per kilogram of solvent, one molal equals one mole of solute.
Since 1 kilogram is equivalent to 1000 milliliters of ethanol, it is possible to compute the number of moles of acetone as follows:
1000 mL/kg times 1.00 mol/kg is 1.00 mol.
The mass of acetone in the solution can then be determined using the molar mass of acetone (58.08 g/mol):
1 mole * 58.08 g/mol = 58.08 g
The volume of acetone can then be calculated by dividing the mass by the density:
58.08 g/0.788 g/mL equals 73.56 mL.
Finally, the molality of acetone can be determined as follows:
0.0074 mol/L (1.00 mol)/1.00 mol/(73.56 mL + 1000 mL) = 0.74 mM.
To know more about acetone, visit:
https://brainly.com/question/13334667
#SPJ4
3. The speed of a reaction can be increased by increasing reactant concentration or decreasing
particle size. *
(1 Point)
True
False
Answers
Answer:
true
because with the both states we increase the surface of reaction
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
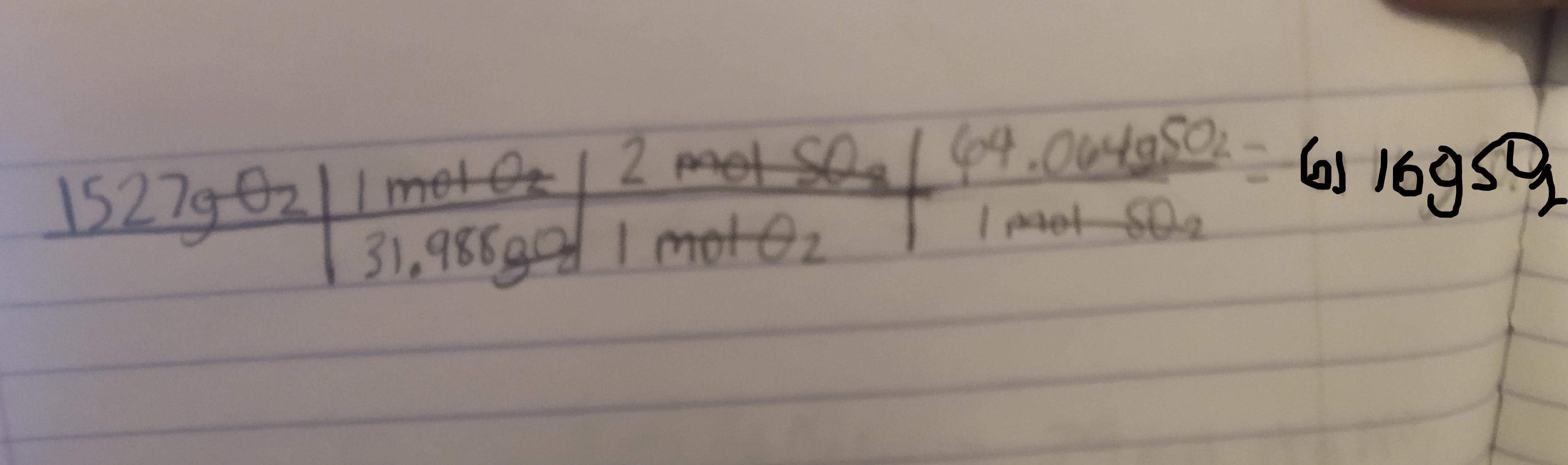
List the 2 pKa's for H2SO4
Answers
What would happen if we had a decrease in greenhouse gases
Answers
Answer:
Investigating delayed detections of climate mitigation benefits arising from individual and combined reductions of climate forcing emissions, the scientists' simulations demonstrated that if we fully stopped greenhouse gas emissions in 2020, warming could be halted as soon as 2033, but this is highly impractical
Explanation:
what might happen if we heat the sphere
Answers
A nearby pond has what appears to be steam coming off of it after a cold front passes through. What is it?
a. evaporation
b. sublimation
c. vaporization
d. condensation
Answers
Answer:
a. evaporation .
Explanation:
In this case, given the described situation, we should take into account that there are two types of liquid-gas phase transitions, evaporation and vaporization, which occur in totally different way.
Firstly, evaporation is a superficial phenomena, it means that it occurs at the surface of the liquid only whereas the vaporization is a bulk phenomena, which means that it occurs along the whole volume of liquid.
In such a way, we can infer that cold steam stream flowing over the pond has the capacity to strip or remove liquid water molecules in the pond and take them to the vapor phase, which means that the answer is a. evaporation .
Best regards.
what is the method of seperating inmisible liquid
Answers
For the element of Sulfur ( S ) answer the following question;
Write the electron configuration of its most common ion.
Answers
A pure copper cube has an edge length of 1.76 cm. How many copper atoms does it contain? (volume of a cube = (edge length)^3; density of copper = 8.96 g/cm^3 )
Answers
Explain why the element of an atom doesn't change if you remove or add electrons or neutrons
Answers
Answer:
If you could miraculously remove two neutrons from an atom's nucleus, the atomic number and electrical charge would stay unaltered. Because neutrons have no electrical charge, adding or removing them from the nucleus has no effect on the nucleus's electrical charge. It does, however, alter the nucleus's mass. Isotopes are formed by adding or subtracting neutrons from the nucleus.
Describe echolocation. Give an example of an animal that uses echolocation.
Answers
dolphin and whales use echolocation