Answers
The increasing order of solubility in water is in the following order. Quinol<Phenol<Resorcinol<Catechol.
Quinol or hydroquinone has the least solublity in the water among given compounds as it has keto-enol tautomerism. Due to this tautomerism, the polar hydroxy groups get converted to keto, this decreases hydrogen bonding capability with water molecules.
Phenol has only one polar hydroxyl group whereas catechol and resorcinol have two hydroxy groups in their structure. So phenol is less soluble in nature than catechol and resorcinol.
On the other hand, Resorcinol has two hydroxyl groups that are in meta position on the benzene ring whereas, in catechol, the hydroxyl groups are ortho position to each other.
In resorcinol, intermolecular hydrogen bonding can be seen between two resorcinol molecules. So hydrogen bonding with water is less leading to a meagre solubility range .
In catechol, the hydroxyl groups are close to each other and there is intermolecular hydrogen bonding between those hydroxyl groups. So no hydrogen bonding could be seen between catechol molecules and thus solubility in water increases.
Therefore, the increasing order of solubility of the compounds would be:
Quinol<Phenol<Resorcinol<Catechol.
To know more about hydrogen bonding, click below:
https://brainly.com/question/1426421
#SPJ9
Related Questions
Which best describes a neutralization reaction?
➪A reaction between an acid and a base
➪A reaction between two acids
➪A reaction between a base and a salt
➪A reaction between two salts
Answers
Answer:
D got it right
Explanation:
Answer:
The answer is d
Explanation:
God bless you have a great day
A 1.555-g sample of baking soda decomposes with heat to produce 0.991 g Na2CO3. Refer to Example Exercise 14.l and show the calculation for the theoretical yield of Na2CO3.
What is the percent yield of sodium carbonate, Na2CO3?
6. A 1473-g unknown mixture with baking soda is heated and has a mass loss of 0.325 g. Refer to Example Exercise 14.2 and show the calculation for the percentage NaHCOs in the mixture.
Answers
Answer:
a) 101%
b)59.7%
Explanation:
The equation for the thermal decomposition of baking soda is shown;
2NaHCO3 → Na2CO3 + H2O + CO2
Number of moles of baking soda= mass/molar mass= 1.555g/84.007 g/mol = 0.0185 moles
From the reaction equation;
2 moles of baking soda yields 1 mole of sodium carbonate
0.0185 moles of baking soda will yield = 0.0185 moles ×1 /2 = 9.25 ×10^-3 moles of sodium carbonate.
Therefore, mass of sodium carbonate= 9.25 ×10^-3 moles × 106gmol-1= 0.9805 g of sodium carbonate. This is the theoretical yield of sodium carbonate.
%yield = actual yield/theoretical yield ×100
% yield = 0.991/0.9805 ×100
%yield = 101%
Since ;
2NaHCO3 → Na2CO3 + H2O + CO2
And H2O + CO2 ---> H2CO3
Hence I can write, 2NaHCO3 → Na2CO3 + H2CO3
Molar mass of H2CO3= 62.03 gmol-1
Molar mass of baking soda= 84 gmol-1
Therefore, mass of baking soda=
0.325/62.03 × 2 × 84 = 0.88 g of NaHCO3
% of NaHCO3= 0.88/1.473 × 100 = 59.7%
The decomposition reaction of baking soda is a reaction in which water and carbon dioxide ae given off as gaseous products.
5. The theoretical yield of Na₂CO₃ is approximately 0.9809 gramsThe percentage yield of sodium carbonate is approximately 101.02%.6. Percentage of NaHCO₃ in the mixture is approximately 59.76%.Reasons:
Mass of baking soda = 1.555 g
Mass of Na₂CO₃ produced = 0.991 g
Required:
Calculation for the theoretical yield
Solution:
Theoretical yield (mass) of Na₂CO₃ produced is found as follows;
Molar mass of Na₂CO₃ = 105.9888 g/mol
Molar mass of NaHCO₃ = 84.007 g/mol
\(\displaystyle 1.555 \, g \, NaHCO_3 \times \frac{1 \, mol \, NaHCO_3}{84.007 \, g \, NaHCO_3} \times \frac{1 \, mol \, Na_2CO_3}{2 \, mol \, NaHCO_3} \times 105.9888 \ g \approx 0.9809 \, g \, Na_2CO_3\)
The theoretical yield of Na₂CO₃ ≈ 0.9809 grams.
The percentage yield is given as follows;
\(\displaystyle Percentage \ yield = \mathbf{\frac{Actual \, Yield}{Theorectical \, Yield} \times 100 \%}\)
The percentage yield of Na₂CO₃ is therefore;
\(\displaystyle Percentage \ yield \ of \ Na_2CO_3= \frac{0.991}{0.9809} \times 100 \% \approx \underline{ 101.02 \%}\)
(Some baking soda may remain if the reaction is not completed)
6. Mass of the unknown mixture of baking soda = 1473 g
Mass loss from the mixture = 0.325 g
Required:
The percentage of NaHCO₃ in the mixture.
Solution:
The chemical in the mass loss from heating the NaHCO₃ = H₂CO₃
Molar mass of H₂CO₃ = 62.03 g/mol
\(\displaystyle \mathrm{Number \ of \ moles \ of \ H_2CO_3 \ produced} = \frac{0.325 \, g}{62.03 \, g/mol} \approx 5.2394 \times 10^{-3} \ moles\)
The chemical reaction is presented as follows;
2NaHCO₃(s) \(\underrightarrow {\Delta \ Heated}\) Na₂CO₃(s) + H₂CO₃(g)2 moles of NaHCO₃ produces 1 mole of H₂CO₃The number of moles of NaHCO₃ in the mixture is therefore;
2 × 5.2394 × 10⁻³ moles ≈ 1.04788 × 10⁻² molesMass of NaHCO₃ in the mixture is therefore
Mass of NaHCO₃ = 1.04788 × 10⁻² moles × 84.007 g/mol = 0.88029 g\(\displaystyle Percentage \ of \ NaHCO_3 \ in \ the \ mixture \ = \mathbf{ \frac{Mass \ of \ NaHCO_3}{Mass \ of \ mixture} \times 100}\)
Which gives;
\(\displaystyle Percentage \ of \ NaHCO_3 \ in \ the \ mixture \ = \ \frac{0.88029 \, g}{1.473 \, g} \times 100 \approx \underline{ 59.76 \%}\)Learn more here:
https://brainly.com/question/21091465
Determine the kinds of intermolecular forces that are present in each element or compound. Part A KrKr Check all that apply. Check all that apply. dispersion forces dipole-dipole forces hydrogen bonding
Answers
Answer:
The kinds of intermolecular forces that are present in each element Kr-Kr.
Explanation:
Since Kr is an inert gas and in atomic form only it is highly stable.
So, Kr gas does not form molecules.
Between the atoms of inert gas, there exist London dispersion forces.
Hence, the intermolecular forces that are present between Kr-Kr atoms is London dispersion forces.
Which of the following balanced reactions does not have at least one 6 as a coefficient?
Responses
H3PO4 + Ba(OH)2 → Ba3(PO4)2 + H2O
H, 3, PO, 4, + Ba(OH), 2, → Ba, 3, (PO, 4, ), 2, + H, 2, O
H2SO4 + Al(OH)3 → Al2(SO4)3 + H2O
H, 2, SO, 4, + Al(OH), 3, → Al, 2, (SO, 4, ), 3, + H, 2, O
C6H12O6 + O2 → CO2 + H2O
C, 6, H, 12, O, 6, + O, 2, → CO, 2, + H, 2, O
C2H2 + O2 → CO2 + H2O
Answers
The following balanced reactions does not have at least one 6 as a coefficient : C₂H₂ + O₂ -----> CO₂ + H₂O
The balanced reaction are as follows :
1) The given reaction is :
H₃PO₄ + Ba(OH)₂ ---> Ba₃(PO₄)₂ + H₂O
balanced reaction is :
2H₃PO₄ + 3Ba(OH)₂ ---> Ba₃(PO₄)₂ + 6H₂O
2) The given reaction is :
H₂SO₄ + Al(OH)₃ ----> Al₂(SO₄)₃ + H₂O
balanced reaction is:
3H₂SO₄ + 2Al(OH)₃ ----> Al₂(SO₄)₃ + 6H₂O
3) The given reaction is :
C₆H₁₂O₆ + O₂ ----> CO₂ + H₂O
balanced reaction is given as :
C₆H₁₂O₆ + 6O₂ ----> 6CO₂ + 6H₂O
4) The given reaction is as follows :
C₂H₂ + O ----> CO₂ + H₂O
balanced reaction is given as follows :
2C₂H₂ + 5O ----> 4CO₂ + 2H₂O
Thus, The following balanced reactions does not have at least one 6 as a coefficient : C₂H₂ + O₂ -----> CO₂ + H₂O
To learn more about balanced reaction here
https://brainly.com/question/28215760
#SPJ1
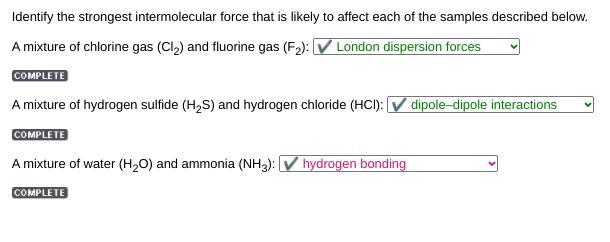
Identify the strongest intermolecular force that is likely to affect each of the samples described below.
A mixture of chlorine gas (Cl) and fluorine gas (F): V London dispersion forces
COMPLETE
Tweaks
Menu
A mixture of hydrogen sulfide (H2S) and hydrogen chloride (HCI): V dipole-dipole interactions
Search
Selection
COMPLETE
Guess
this
hydrogen bonding
A mixture of water (H2O) and ammonia (NH3):
Answers
Answer:
A mixture of chlorine gas (Cl2) and fluorine gas (F2):
✔ London dispersion forces
Explanation:
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium? CO(g) + Cl2(8)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of Cl2(g) present is approximately 347.37 mol.
To determine the number of moles of Cl2(g) at equilibrium, we need to use the given equilibrium constant (Kc) and set up an ICE table to track the changes in the reactants and products.
The balanced equation for the reaction is:
CO(g) + Cl2(g) ⇌ COCl2(g)
Let's set up the ICE table:
CO(g) + Cl2(g) ⇌ COCl2(g)
Initial: 0.3500 0.05500 0
Change: -x -x +x
Equilibrium: 0.3500 - x 0.05500 - x x
Using the equilibrium concentrations in the ICE table, we can write the expression for the equilibrium constant (Kc) as:
Kc = [COCl2(g)] / [CO(g)][Cl2(g)]
Substituting the values into the equation, we have:
1.2 × 10^3 = (0.05500 - x) / [(0.3500 - x)(0.05500 - x)]
Simplifying the equation, we can cross-multiply and rearrange:
1.2 × 10^3 × (0.3500 - x)(0.05500 - x) = 0.05500 - x
Expanding and rearranging, we get:
0 = (1.2 × 10^3 × 0.05500 - 1.2 × 10^3x + 0.05500x) - x
Simplifying further:
0 = 66 - 1.245x + 0.05500x - x
0 = 66 - 0.19x
0.19x = 66
x = 66 / 0.19
x ≈ 347.37
For more such questions on equilibrium visit:
https://brainly.com/question/19340344
#SPJ8
14) A wavelength of radiation has a frequency of 2.10 x 1014 Hz. What is the wavelength of this radiation in nm, and
determine the type of radiation.
Answers
Answer:
λ = 1.43 x 10³ meters (radio waves)
Explanation:
c = f·λ => λ = c/f
λ = wavelength = ?
f = frequency = 2.10 x 10¹⁴ Hz = 2.10 x 10¹⁴ cycles/sec
c = speed of light (vacuum) = 3.0 x 10⁸m/sec
λ = c/f = 3.0 x 10⁸m/sec / 2.10 x 10¹⁴sec⁻¹ = 1.43 x 10³ meters (radio waves)

The wavelength of this radiation in nm: is equal to 1430 nanometers.
Given the following data:
Frequency = \(2.10 \times 10^{14}\) HzWe know that the speed of a radiation is equal to \(3.0 \times 10^8\) meters.
To determine the wavelength of this radiation in nm:
Mathematically, the wavelength of a waveform is calculated by using the formula;
\(\lambda = \frac{v}{f}\)
Where:
f is the frequency of a wave.v is the speed of light.Substituting the given parameters into the formula, we have;
\(\lambda = \frac{3.0 \times 10^8}{2.10 \times 10^{14}} \\\\\lambda = 1.43\times 10^{-6} \;meters\)
In nanometer:
\(\lambda = 1.43 \times 10^{-6} \times 10^{9} \\\\\lambda =1430\;meters\)
Wavelength = 1430 nanometers.
On the electromagnetic spectrum, the type of radiation with a wavelength of 1430 nanometers is an infrared radiation.
Read more: https://brainly.com/question/14708169
Trend of atomic number and atomic size of the elements when we move from left to right in different periods of periodic table
Answers
Answer:
The atomic size decreases with an increase in atomic number when we move from left to right.
Explanation: Hope it helps you:))))))
Have a great day.
How many grams of lead will be produced if 2.54g of PbS is burned with 1.88g of O2? write the equation
Answers
If 2.54 g of PbS is burned with 1.88 g of O2, approximately 2.20 grams of Pb will be produced.
The balanced equation for the reaction of lead sulfide (PbS) with oxygen (O2) to produce lead (Pb) and sulfur dioxide (SO2) is as follows:
2PbS + 3O2 -> 2Pb + 2SO2
From the balanced equation, we can see that the stoichiometric ratio between PbS and Pb is 2:2 or 1:1. This means that for every 1 mole of PbS, 1 mole of Pb is produced.
To calculate the number of moles of PbS, we need to divide the given mass (2.54 g) by its molar mass:
Molar mass of PbS = 207.2 g/mol (Pb) + 32.07 g/mol (S) = 239.27 g/mol
Moles of PbS = 2.54 g / 239.27 g/mol = 0.0106 mol
Since the stoichiometric ratio between PbS and Pb is 1:1, the number of moles of Pb produced is also 0.0106 mol.
To calculate the mass of Pb, we multiply the number of moles by its molar mass:
Molar mass of Pb = 207.2 g/mol
Mass of Pb = 0.0106 mol x 207.2 g/mol = 2.20 g
This calculation is based on the stoichiometric ratio between PbS and Pb, where 1 mole of PbS produces 1 mole of Pb. By converting the given mass of PbS to moles and then multiplying by the molar mass of Pb, we can determine the mass of Pb produced.
For more such question on PbS. visit :
https://brainly.com/question/27964828
#SPJ8
The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balanced OXIDATION half reaction.
Bi(OH)3 + NO2 → Bi + NO3-
Answers
Answer:
\(N^{4+}O_2+2OH^-\rightarrow (N^{5+}O_3)^-+1e^-+H_2O\)
Explanation:
Hello,
In this case, for the given reaction, we first start by the writing of the oxidation states of all the involved elements:
\(Bi^{3+}(OH)^-+N^{4+}O^{2-}_2\rightarrow Bi^0+(N^{5+}O^{2-}_3)^-\)
In such a way, we are noticing nitrogen is undergoing an increase in its oxidation state, therefore it is being the oxidized species, for which the oxidation half reaction, should be (considering basic conditions):
\(N^{4+}O_2+H_2O+2OH^-\rightarrow (N^{5+}O_3)^-+1e^-+2H^++2OH^-\\\\N^{4+}O_2+H_2O+2OH^-\rightarrow (N^{5+}O_3)^-+1e^-+2H_2O\\\\N^{4+}O_2+2OH^-\rightarrow (N^{5+}O_3)^-+1e^-+H_2O\)
Best regards.
HELP PLSSS
Why is the water cycle important for ecosystems?
A. The water cycle helps plants keep and store water. The cycle makes sure that water stays at earth's surface and is available to ecosystems.
B. The water cycle photosynthesis to make clouds. These clouds make surface water that animals drink to survive in there ecosystem.
C. The water cycle permanently removes water from the underground and from large bodies of water and transports it to terrestrial ecosystems
D. The water cycle helps recycle and transport water around the planet. The cycle replenishes the water supply of ecosystems.
Answers
Answer:
The water cycle is often taught as a simple circular cycle of evaporation, condensation, and precipitation. Although this can be a useful ...
Explanation:
Answer: Your answer will be D)
Explanation: Hope this helped
Pick the selection that increases in energy.
7f, 6s, 4p
4s, 4p, 4d
1s, 6f, 4d
3d, 4s, 3p
Answers
Answer:
i think mix im um 1s 6f 4d is correct answer
20 points WILL GIVE BRAINLIEST
1. Which statement is INCORRECT?
A. Aristarchus calculated the distances between the sun and
earth fairly accurately.
B. Aristarchus underestimated the size of the sun in relation
to earth.
C. Aristarchus's estimates of the relative sizes of the earth
and moon were not too far off.

Answers
Answer:
The answer is A.
Explanation:
Aristarchus largely underestimated the size of the sun, but he accurately measured the relative size of the earth to the moon
4. Describe how you will measure the volume of a piece of stone
Answers
Answer:
One way to measure the volume of any irregular object (in your case, a stone) is to submerge it completely under water and measure the change in the height of the water level. This change in the water level (let's say it goes from 50 mL to 65 mL) indicates that the stone has a volume of 15 mL.
A beaker contains a total of 500 ml of solution which is 0.00050 M Ag^+, 0.00050 M Pb^2+, and 0.00050 M in Mn^2+ ions. If 10.00 ml of 1.0*10^-6 M Na2CO3 is added to the beaker, what will precipitate?
Ksp Ag2CO3 = 8.1*10^-12
Ksp PbCO3 = 7.4*10^-14
Ksp MnCO3 = 8.8*10^-11
Answers
Only Ag2CO3 will precipitate from the solution.
Precipitation reactionWhen Na2CO3 is added to the solution, it will react with the Ag^+ and Pb^2+ ions to form precipitates of Ag2CO3 and PbCO3. The Mn^2+ ion concentration is not high enough to form a precipitate with Na2CO3.
First, let's calculate the initial concentration of Ag^+ and Pb^2+ ions in the solution:
Ag^+: 0.00050 M
Pb^2+: 0.00050 M
Next, we need to calculate the concentration of Na2CO3 after it is added to the solution. Since we added 10.00 ml of 1.0*10^-6 M Na2CO3 to a total volume of 500 ml, the final concentration of Na2CO3 is:
[Na2CO3] = (10.00 ml / 500 ml) * 1.010^-6 M
[Na2CO3] = 2.010^-8 M
Now we can use the Ksp values to determine which precipitates will form.
For Ag2CO3:
Ksp = [Ag^+]^2[CO3^2-]
8.110^-12 = (2x)^2 (2x)
8.110^-12 = 4x^3
x = 2.0*10^-4 M
Since the concentration of CO3^2- is higher than the solubility product, Ag2CO3 will precipitate.
For PbCO3:
Ksp = [Pb^2+][CO3^2-]
7.410^-14 = (0.00050 M)(2x)
x = 9.210^-11 M
Since the concentration of CO3^2- is lower than the solubility product, PbCO3 will not precipitate.
Therefore, the only precipitate that will form is Ag2CO3.
More on precipitation reactions can be found here: https://brainly.com/question/24158764
#SPJ1
The pH of an acidic solution is 4.83. What is [H"]?
Answers
\(pH = -\log[H^{+}] \\\\\implies \log[H^{+}] = -pH\\\\\implies [H^{+}] = 10^{-pH}\\\\\implies [H^{+}] = 10^{-4.83} = 0.000015\)
If you produced 5 moles of Ca3(PO4)2, how many moles of NaCl did you also produce?
Answers
15 moles of NaCl would have been used up in the reaction as a result.
What volume of Ca3 PO4 2 will be generated?The number of moles is obtained by taking each mass and dividing it by the molar mass. The molar mass of Ca₃(PO₄)₂ is 310.18 grammes/mole. The result is 0.016 moles of Ca₃(PO₄)₂ when five is divided by 310.18.
The balanced chemical equation for the reaction of sodium chloride (NaCl) and calcium phosphate (Ca₃(PO₄)₂) is:
3Ca₃(PO₄)₂ + 6NaCl → 6NaPO₄ + CaCl₂ + 2Ca₃(PO₄)₂
calculate the amount of NaCl consumed using the following proportion:
2 mol Ca₃(PO₄)₂ / 6 mol Sodium Chloride = 5 mol Ca₃(PO₄)₂ / x
Solving for x, we get:
x = (6 mol NaCl × 5 mol Ca₃(PO₄)₂) / 2 mol Ca₃(PO₄)₂
x = 15 mol NaCl
To know more about reaction visit:-
brainly.com/question/28984750
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Solve for the missing values?
Help me plz!

Answers
Substituting this in to the angle 7x (which is equal to y), we get y= 84 degrees.
We also see that 24 + y + x = 180 since it is along a straight line. So 24 + 84 + z = 180 and then z = 72 degrees.
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1
What mass of sodium hydroxide will completely neutralize 2.5 mol of sulfuric acid?
Answers
Given :
2.5 mole of Sulfuric acid \(( H_2SO_4 )\) .
To Find :
Mass of sodium hydroxide will completely neutralize 2.5 mol of sulfuric acid
Solution :
Let us assume volume of water be 1 L .
Now , we know , to neutralize 1 mole of sulfuric acid we need 2 moles of NaOH .
So , for 2.5 mole sulfuric acid required 5 mole of NaOH .
Moles of NaOH ,
\(n=M\times Volume \\\\n=5\times 1=5\ moles\)
Molecular mass of NaOH , M.M = 58.44 g/mol .
Mass of 5 moles of NaOH :
\(m=5\times 58.44\ g\\\\m=292.2\ g\)
Hence , this is the required solution .
Identify if the following is an intensive or extensive property.
The string is 3 meters long
Answers
Answer:
This is Extensive object because intensive properties do not depend on the quantity of matter. Examples include density, state of matter, and temperature. Extensive properties do depend on sample size. Examples include volume, mass, and size
A student measures the molar solubility of silver sulfite in a water solution to be 1.53x10-5M. Based on her data , the solubility product constant for this compound is

Answers
ANSWER:
EXPLANATION
Given that:
\(\text{ The molar solubility of silver sulfide is 1.53 x 10}^{-5}\)To find the solubility constant product for the compound, follow the steps below
Step 1: Write the ionic equation for silver sulfite
\(Ag_2SO_3\rightarrow\text{ 2Ag}^+\text{ + SO}_3^{2-}\)From the above reaction, the silver sulfite split into silver ion and sulfite ion
\(undefined\)How many atoms are in a sample containing 4.000 moles of carbon?
A)6.022 x 10
23
atoms
B)2.408 x 10
24
atoms
O
24
C)7.233 x 10 atoms
D)2.893 x 10
25
atoms
Answers
Answer:
c I think but that's only what i think
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 24.088×10²³ atoms of carbon are in sample containing 4.000 moles of carbon. The correct option is option B.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
number of atoms/molecules/ formula units of carbon= number of moles of carbon × 6.022×10²³
number of moles of carbon= 4 moles
substituting all the given values in the above equation, we get
number of atoms/molecules/ formula units of carbon= 4 × 6.022×10²³
number of atoms/molecules/ formula units of carbon=2.4088×10²⁴ atoms of carbon
There are 2.4088×10²⁴ atoms of carbon
Therefore, 24.088×10²³ atoms of carbon are in sample containing 4.000 moles of carbon. The correct option is option B.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ6
An atom has 12 protons, 11 neutrons and 12 electrons. What is the mass member of this atom?
Answers
Answer:
the mass is 24
Explanation:
The atomic number is defined as the totall number of electons in the atom in its non bonded state.
Thus the number of electron is 1w, atomic number is also 12.
Mass number is defined as the number of protons + neutrons in the nucleus of an atom.
A combination reaction is given below: 3A 2B --5C If compound A has a molar mass of 159.7 g/mole and compound C has a molar mass of 57.6 g/mole, how many grams of compound C will be produced from 26.96 grams of compound A and excess compound B
Answers
Answer:
16.2 g
Explanation:
Step 1: Write the balanced reaction
3 A + 2 B ⇒ 5 C
Step 2: Calculate the moles corresponding to 26.96 g of A
The molar mass of A is 159.7 g/mol.
\(26.96 g \times \frac{1mol}{159.7g} = 0.1688 mol\)
Step 3: Calculate the moles of C produced from 0.1688 moles of A
The molar ratio of A to C is 3:5. The moles of C produced are 5/3 × 0.1688 mol = 0.2813 mol.
Step 4: Calculate the mass corresponding to 0.2813 moles of C
The molar mass of C is 57.6 g/mol.
\(0.2813 mol \times \frac{57.6g}{mol} = 16.2 g\)
At a certain temperature, the equilibrium constant, c,
for this reaction is 53.3.
H2(g)+I2(g)↽−−⇀2HI(g)c=53.3
At this temperature, 0.400 mol H2
and 0.400 mol I2
were placed in a 1.00 L container to react. What concentration of HI
is present at equilibrium?
Answers
At equilibrium, the HI concentration is 2.92 mol/L.
What is the H2 CO2 reaction's equilibrium constant?For the process H2(g)+CO2(g)updownarrow H2O+CO. at 1660 °C, the equilibrium constant KP is 4. In a 5 liter flask, 0.8 moles each of H2 and CO2 are first injected. Reactions occur in the following order with relation to H2: 1. In experiments 1 and 2, the reaction rate doubles when the initial concentration of H2 is doubled while the initial concentration of Cl2 is held constant.
H2(g) + I2(g) ⇌ 2HI(g)
c = [HI]² / [H2][I2]
At the specified temperature, c = 53.3, hence the following can be written:
53.3 = [HI]^2 / (0.400 mol/L) × (0.400 mol/L)
or, [HI]² = 53.3 × 0.16
or, [HI]² = 8.528
or, [HI] = sqrt(8.528) mol/L
or, [HI] = 2.92 mol/L
As a result, 2.92 mol/L of HI are present at equilibrium.
To know more about concentration visit:-
https://brainly.com/question/10725862
#SPJ1
Is the study of matter and energy complete, or do you think there’s still new information to discover?
Answers
Answer:
Physical science, in turn, can be divided into chemistry and physics. Chemistry is the study of matter and energy at the scale of atoms and molecules. ... Physics is the study of matter and energy at all scales—from the tiniest particles of matter to the entire universe but i dont really know if there is new information to discover
Explanation:
hope it help and if it doesnt sorry ;)
What is the correct conversion factor when converting from moles to liters?
Answers
The correct conversion factor when converting from moles to liters is the molar volume at a given temperature and pressure.
This value is dependent on the ideal gas law and can be determined using the ideal gas equation PV = nRT, where P represents pressure, V represents volume, n represents the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
The molar volume of an ideal gas at standard temperature and pressure (STP), which is 0 degrees Celsius (273.15 Kelvin) and 1 atmosphere (101.3 kilopascals), is approximately 22.4 liters per mole.
Therefore, to convert moles to liters, you can multiply the number of moles by the molar volume at STP, which gives you the volume in liters.
It's important to note that the molar volume is an approximation and assumes ideal gas behavior.
Additionally, if you are working with gases at different temperatures and pressures, you would need to use the appropriate molar volume value corresponding to the given conditions.
for such more questions on volume
https://brainly.com/question/29796637
#SPJ8
Mrs. Elder travelled from Zillah, WA to Tacoma, WA in 3 hours. She traveled 201 miles on the trip. What was the speed she traveled at with no stops? (Speed=Distance/Time)
Answers
Answer:
201 ÷ 3 = 67mph
Explanation:
let me know if you want me to explain more