Answers
The equation of this straight line is equal to y = -1/2x + 1.
How to calculate the equation of the given line?First of all, we would determine the slope of the straight line by using this formula:
\(Slope,\;m = \frac{Change\;in\;y\;axis}{Change\;in\;x\;axis}\\\\Slope,\;m = \frac{y_2\;-\;y_1}{x_2\;-\;x_1}\)
By critically observing the graph, the points on the x-axis and y-axis is given by:
Points (x₁, x₂) = (0, 2)
Points (y₁, y₂) = (1, 0)
Substituting the given points into the formula, we have;
Slope, m = (0 - 1)/(2 -0)
Slope, m = -1/2
In Mathematics, the y-intercept of any graph occurs at the point where the value of "x" is equal to zero (0).
y-intercept = (0, 1)
Mathematically, the standard form of the equation of a straight line is given by;
y = mx + c
Where:
x and y are the points.m is the slope.c is the intercept.Substituting the parameters into the formula, we have;
y = -1/2x + 1.
Read more on slope here: https://brainly.com/question/11586413
#SPJ1
Related Questions
Determine the name or formula for each polyatomic ion.
formula: PO3−4
name:
name: sulfite ion formula:
name: sulfate ion formula:

Answers
Answer:
See explanation
Explanation:
PO4{3-} is phosphate
Sulfite's formula is SO3{2-}
Sulfate is SO4{2-}
OH- is hydroxide
Note: {x±} signifies the charge of the entire molecule
The polyatomic ions in question are phosphite ion, sulfite ion, and sulfate ion.
Explanation:The formula PO3−4 represents the polyatomic ion called phosphite ion. It is composed of one phosphorus atom bonded to three oxygen atoms. The name of the sulfite ion is SO3−2, and it consists of one sulfur atom bonded to three oxygen atoms. Lastly, the sulfate ion has the formula SO4−2, and it is composed of one sulfur atom bonded to four oxygen atoms.
Learn more about Polyatomic ions here:https://brainly.com/question/35456287
#SPJ6
Which of the following observations about pH is consistent for an acidic solution?
A pH value of 100
or
A pH value of 3
or
A pH value of 7
or
A pH value of 10
Answers
Answer:
A pH value of 3
Explanation:
The pH scale has 14 numbers:
- 7 is a true natural
-anything above 7 is a base
-and anything under 7 is an acid
pH of three is a strong acid
Why is it necessary to cool a hot , supersaturated solution slowly during a recrystallization?
Answers
Answer:
This is because, the is less chance of trapping impurities in the developing crystal lattice
Answer:
injuries
Explanation:
bc it will burn your hands
two uses of sodium carbonate
Answers
Sodium carbonate, also known as washing soda or soda ash, has a wide range of applications. Sodium carbonate can be naturally occurring or synthetically produced through various methods, including the Solvay process, which is the most common method of industrial production.
Sodium carbonate, also known as washing soda or soda ash, has many uses, including:
1) Cleaning agent: Sodium carbonate is an effective cleaning agent due to its alkaline nature. It is used in laundry detergents and household cleaners to remove stains and grease from clothes and surfaces.
2) Industrial applications: Sodium carbonate is used in a variety of industrial applications. It is used in the production of glass, pulp and paper, and soaps and detergents. It is also used as a water softener and pH regulator in chemical processes.
Learn more about Sodium Carbonate at
brainly.com/question/31344166
#SPJ1
Gold's natural state has a definite shape and a definite volume. What is gold's natural state(s)?
Answers
Answer:
If your asking what golds natural state of matter is it's solid.
Explanation:
Answer:
the answer is soild
Explanation:
i did it on edge :)
What is the number of moles in 546 g strontium nitrite?
A. 0.388 mol
B. 2.58 mol
C. 329 mol
D. 3.04 mol
Answers
3. Does matter increase in
mass when mixed with
another substance?
a Yes
b. No
Answers
Answer:
b. No
Explanation:
The question above is related to "The Law of Conservation of Mass." This law states that mass is neither created nor can it be destroyed. Even if a particular matter will be mixed with another substance or it undergoes chemical reaction, the mass of the matter will remain the same. Even with a change in phase, the mass of the matter will remain the same.
How much energy is required to vaporize 2 kg of copper? Use the table below
and this equation: Q = mLvapor-
A. 414 kJ
B. 9460 kJ
C. 4730 kJ
D.207 kJ

Answers
How much heat (in joules) does it take to heat 180 g of water from 24 °C to 66 °C? The heat capacity of water is 4.184 J/(g · °C). You may round your answer to the nearest 100 J
Answers
Answer:31,631.04
Explanation: multiply final temp with initial temp = 42 then multiplied by grams = 7,560 then multiplied by J = 31,631.04
PLEASE HELP ASAP!
5 + 6 HNO3 -> H2504 + 6 NO2 + 2H20
In the above equation how many moles of water can be made when 112.6 grams of HNO3 are consumed?
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Hydrogen 1
Nitrogen 14
Sulfur 32
Oxygen 16
Answers
0.595 moles of water can be made at 112.6 grams of \(HNO_{3}\) are consumed
To determine the number of moles of water produced when 112.6 grams of \(HNO_{3}\) are consumed, we use the equation's stoichiometry and molar masses.
To determine the number of moles of water produced when 112.6 grams of \(HNO_{3}\) are consumed, we need to use the molar mass of \(HNO_{3}\) and the stoichiometric coefficients of the balanced chemical equation.
The molar mass of \(HNO_{3}\) is calculated as follows:
1 mole of hydrogen (H) = 1 g/mol
1 mole of nitrogen (N) = 14 g/mol
3 moles of oxygen (O) = 3 × 16 g/mol = 48 g/mol
Adding these together, the molar mass of \(HNO_{3}\) is 1 + 14 + 48 = 63 g/mol.
Now, we can set up a conversion factor using the stoichiometry of the balanced equation:
From the equation: 5 + 6 \(HNO_{3}\) -> \(H_{2}SO_{4}\) + 6 \(NO_{2}\) + 2 \(H_{2}O\)
From the coefficients: 6 moles of \(HNO_{3}\) produce 2 moles of \(H_{2}O\)
To find the moles of water produced, we use the following calculation:
112.6 g \(HNO_{3}\) × (1 mol \(H_{2}O\) / 63 g \(HNO_{3}\)) × (2 mol \(H_{2}O\) / 6 mol \(HNO_{3}\)) = 0.595 mol \(H_{2}O\)
Therefore, when 112.6 grams of \(HNO_{3}\) are consumed, approximately 0.595 moles of water can be produced according to the given balanced equation and molar masses.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ8
Softening of water is the application of
Answers
Answer:
Water softening is the process of removing the dissolved calcium and magnesium salts that cause hardness in water
Explanation:
The approximate diameter of the spherical nucleus of hydrogen-1 atom is 1 x 10^-13 cm. Estimate the density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24
Answers
The diameter of the spherical nucleus of hydrogen-1 atom is 1 x 10^-13 cm. The density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24 is 3.193 × 10¹⁵ g/cm³.
What is density ?
The density of a substance is its mass per unit volume. The most common symbol for density is, but the Latin letter D can also be used. Density is defined mathematically as mass divided by volume.
Diameter = 1 x 10⁻¹³ cm
Mass = 1.673 x10⁻²⁴ g
Volume = 4/3πr³
Volume = 4/3 × 22/7 × (1 × 10⁻¹³ / 2)³
Volume = 11 × 10⁻³⁹ / 21
Density = Mass / Volume
= 1.673 x10⁻²⁴ / 11 × 10⁻³⁹ / 21
Density = 3.193 × 10¹⁵ g/cm³
Thus, the density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24 is 3.193 × 10¹⁵ g/cm³.
To learn more about the density, follow the link;
https://brainly.com/question/29775886
#SPJ9
environmental conditions of the ocean
Answers
The environmental conditions of the ocean are:
Light availabilityOxygen levelsWater movementSalinityDensity and pH.What are the environmental impacts on the ocean?In our oceans, there are known to be a lot of environmental conditions that are known to often affect the growth, survival and also the productivity of marine organisms. These are the ones listed above.
Note that the increased concentration of chemicals, e.g. nitrogen and phosphorus is one that can be harmful to marine life.
Hence, The environmental conditions of the ocean are:
Light availabilityOxygen levelsWater movementSalinityDensity and pH.Learn more about ocean from
https://brainly.com/question/25154137
#SPJ1
? Is a scientist who is studying cancer with the
goal of finding an effective treatment doing
pure research or applied research?
Answers
A scientist who is studying cancer with the goal of finding an effective treatment is doing applied research.
Applied research refers to scientific investigations that are conducted with the specific purpose of solving practical problems or improving existing technologies. In the case of cancer research, the scientist is aiming to find a treatment that can be directly applied to patients in order to combat the disease.
In contrast, pure research, also known as basic or fundamental research, is conducted to gain a deeper understanding of a particular phenomenon without any immediate practical application in mind. Pure research aims to expand scientific knowledge and explore new theories or concepts. While pure research may indirectly contribute to the development of applied research, its primary goal is to advance our understanding of the natural world.
In summary, a scientist studying cancer with the goal of finding an effective treatment is engaged in applied research, as they are conducting research with the intention of solving a specific problem and developing practical solutions for the benefit of cancer patients.
Learn more about applied research,here:
https://brainly.com/question/11041802
#SPJ3
The initial water level in a 10-mL graduated cylinder reads 4.5mL, after a ruby gemstone is dropped into the cylinder, the water level is read 7.0 mL, What is the volume of the ruby?
Answers
3.0 mL is the volume of the ruby. The volume of a container is commonly believed to be its capacity.
What is volume?Volume is a three-dimensional spatial measurement. It is frequently mathematically quantified using SI-derived units or different imperial or The us customary units. Volume is connected to the notion of length (cubed).
The volume of a container is commonly believed to be its capacity; that is, the quantity of fluid (gas or liquid) that perhaps the container can hold, instead of the amount of space that the container occupies.
volume of water = 4.1 mL
volume of water + the volume of the ruby gemstone = 7.1 mL
volume of the ruby gemstone can be calculated as:
7.1 mL - 4.1 mL = 3.0 mL
Therefore, 3.0 mL is the volume of the ruby.
To learn more about volume, here:
https://brainly.com/question/13807002
#SPJ9
2 Na2O2 + 2 H₂O = 4NaOH+O2
If sodium peroxide, Na2O2, is added to water, elemental oxygen gas, O2, and sodium
hydroxide, NaOH, are generated. Suppose 8.52 g of sodium peroxide, Na2O2, is added to a large
excess of water. What volume, in liters, of oxygen, O2, will be produced?
Answers
The volume of O₂ produced when 8.52 g of Na₂O₂ is added to excess water is approximately 1.17 liters at STP.
What is ideal gas law?When it comes to the ideal gas law, the underlying assumption is that the gas is in a state of thermodynamic equilibrium. This means that its molecules are not interacting with each other except through perfectly elastic collisions.
Equation:
We can use stoichiometry to determine the volume of oxygen gas produced from the reaction of 8.52 g of Na₂O₂.
First, we need to convert the mass of Na₂O₂ to moles using its molar mass:
8.52 g Na₂O₂ × (1 mol Na₂O₂ / 77.98 g Na₂O₂) = 0.1093 mol Na₂O₂
According to the balanced chemical equation, 1 mole of Na₂O₂ produces 1/2 mole of O₂:
2 Na₂O₂ + 2 H₂O → 4 NaOH + O₂
1 mol Na₂O₂ produces 1/2 mol O₂
So, the number of moles of O₂ produced is:
0.1093 mol Na₂O₂ × (1/2 mol O₂ / 1 mol Na₂O₂) = 0.05465 mol O₂
Finally, we can use the ideal gas law to determine the volume of O₂ produced at standard temperature and pressure (STP), which is defined as 0°C (273 K) and 1 atmosphere (1 atm) of pressure:
PV = nRT
At STP, the pressure and temperature are known, so we can rearrange the equation to solve for V:
V = nRT / P
Plugging the values, we get:
V = (0.05465 mol) × (0.08206 L atm mol⁻¹ K⁻¹) × (273 K) / (1 atm)
V ≈ 1.17 L
To know more about ideal gas law, click here
https://brainly.com/question/28257995
#SPJ1
how does a spectral signature help scientists determine the composition of planets and other stars???
Answers
Answer:
How do scientists determine the chemical compositions of the planets and stars? Each element absorbs light at specific wavelengths unique to that atom. When astronomers look at an object's spectrum, they can determine its composition based on these wavelengths. ... That fingerprint often appears as the absorption of light.
Explanation:
We can conduct different spectroscopic techniques for the sample collected from stars and planets to determine their composition. Using these techniques, scientists can identify the compounds and elements present in them.
What are spectroscopic techniques ?There are various spectroscopic techniques such as IR spectroscopy, UV- visible spectroscopy, mass spectroscopy etc. to determine the composition of both inorganic and organic compounds.
The IR spectroscopy uses the vibrational transitions of molecules by the absorption of IR radiation. The stretching or bending frequency of each functional groups are characteristic to them. Hence, we can determine the functional groups present in the molecules.
Similarly mass spectrum, atomic emission spectrum etc. provide valuable information about the sample under study including their mass and elemental compositions. Hence, spectral signature definitely helpful to determine the composition in stars and planets.
Find more on spectroscopy:
https://brainly.com/question/30256292
#SPJ6
What is cracked open to form pentane (C5H12). propene (C3H6), and ethene (C₂H4)?
A. Decane (C10H₂2)
B. Octane (Cs His)
C. Dodecane (C12H26)
D. Nonane (CH₂)
Answers
The compound that was cracked is Decane (C10H₂2). Option A
What is cracking?The term cracking refers to the process by which a heavier hydrocarbons is broken down in order to obtain lighter hydrocarbons. The kinds of cracking that we have are;
1) catalytic cracking
2) Thermal cracking
When we crack up a compound, we can get smaller fractions. Usually, the lighter fractions of a hydrocarbon are much more useful than the heavier fractions this informs the reason why we usually crack up hydrocarbons.
Now, we know that compounds that were obtained from the cracking are; pentane (C5H12). propene (C3H6), and ethene (C₂H4). Thus the compound that was cracked is Decane (C10H₂2). Option A
Learn more about cracking:https://brainly.com/question/13565152
#SPJ1
What information does an equilibrium constant give about a reaction?
O A. It tells how long it takes the reaction to reach equilibrium.
B. It tells whether products or reactants are favored at equilibrium.
C. It tells how much energy is required for the reaction to happen.
O D. It tells what the rate constant of the reaction is at equilibrium.
Answers
Answer:
B. It tells whether products or reactants are favored at equilibrium.
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Plants need to use the gas CARBON DIOXIDE for photosynthesis. true or false
Answers
Pressurized metal gas cylinders are generally used to store commonly used gases in the laboratory. At times, it can be easier to chemically prepare occasionally used gases. For example, oxygen gas can be prepared by heating KMnO4(s) according to the following chemical reaction:
2KMnO4(s) → K2MnO4(s) + MnO2(s) + O2(g)
How many grams of KMnO4 would you need to produce 0.27 moles of O2, assuming 100% conversion?
Answers
Answer:
You need 85.32 grams of KMnO₄ to produce 0.27 moles of O2, assuming 100% conversion.
Explanation:
The balanced chemical reaction is:
2 KMnO₄ (s) → K₂MnO₄ (s) + MnO₂ (s) + O₂ (g)
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles participate in the reaction:
KMnO₄: 2 molesK₂MnO₄: 1 moleMnO₂: 1 moleO₂: 1 moleThen you can apply the following rule of three: if by stoichiometry 1 mole of O₂ is produced by 2 moles of KMnO₄, 0.27 moles of O₂ are produced by how many moles of KMnO₄?
\(moles of KMnO_{4} =\frac{0.27 moles of O_{2} *2moles of KMnO_{4} }{1mole of O_{2} }\)
moles of KMnO₄= 0.54
The molar mass of KMnO₄ is 158 \(\frac{g}{mol}\).
Then the amount of mass present in 0.54 moles of the compound can be calculated by:
0.54 moles* 158.034 \(\frac{g}{mol}\)= 85.32 grams
You need 85.32 grams of KMnO₄ to produce 0.27 moles of O2, assuming 100% conversion.
A gas is in a flexible container (volume can change but no gas can escape) at constant pressure. If 1 liter of gas at 300 K is cooled to 100 K (and assuming that it does not condense to a liquid!), what is the volume (in liters) of the gas at 100 K?
Answers
The volume of the gas at 100K If 1 liter of gas at 300 K is cooled to 100K is 0.33L.
How to calculate volume?The volume of a gas can be calculated using the following formula:
V1/T1 = V2/T2
Where;
V1 = initial volumeV2 = final volumeT1 = initial temperatureT2 = final temperatureAccording to this question, a gas is in a flexible container. If 1 liter of gas at 300K is cooled to 100K. The volume of the gas is calculated as follows:
1/300 = V2/100
100 × 1 = 300V2
100 = 300V2
V2 = 100/300
V2 = 0.33L
Therefore, the volume of the gas at 100K If 1 liter of gas at 300 K is cooled to 100K is 0.33L.
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
How many mg are in 68.3cg
Answers
Answer:10
Explanation:
Determine the amount of moles of SiO2 needed to produce 4.80g of CO.
SiO2(s) + _C(s) — _SiC(s) + I co(g)
A)134 mol
B)0.171 mol
C)67.2 mol
D) 0.0857 mol
Answers
Row 1: 0.8 x 10 0.9 x 10 0.3 x 10 0.6 x 10
Row 2: 0.2 x 10 0.6 x 10 0.2 x 10 0.4 x 10
Row 3: 8.0 2.4 x 10 8.0 1.6 x 10
Row 4: 2.8 8.4 2.8 5.6
Row 5: 0.816 2.45 0.816 1.63
What are the Relative Speed & distance of atoms depending on the state of matter?
Answers
Answer:
Explanation:
The relative speed and distance of atoms in a substance depend on the state of matter.
In a solid, atoms are closely packed together and have minimal movement or vibration. Their relative speed is low and their distance from one another is short.
In a liquid, atoms are still close together but have more movement and freedom of movement than in a solid. Their relative speed is slightly higher than in a solid, and their distance from one another is slightly longer.
In a gas, atoms are widely spaced and have a high degree of movement and freedom of movement. Their relative speed is much higher than in a solid or liquid, and their distance from one another is much greater.
In a plasma, atoms are ionized and have a high degree of movement and freedom of movement. Their relative speed is much higher than in a solid, liquid or gas, and their distance from one another is much greater.
Adding thermal energy to a gas at constant pressure will cause...
A. the volume of the gas to decrease.
B. the volume of the gas to increase.
C. the temperature of the gas to decrease.
D. the pressure of the gas to decrease.
Answers
B. the volume of the gas to increase.
What is thermal energy to a gas at constant pressure?Thermal energy is the energy associated with the random motion of the particles in a substance, such as a gas. When thermal energy is added to a gas at constant pressure, the increased kinetic energy of the gas particles causes them to move apart, increasing the volume of the gas. This relationship is described by Gay-Lussac's Law, which states that at a constant pressure, the volume of a gas is directly proportional to its temperature.
Learn more about Thermal energy in brainly.com/question/18989562
#SPJ1
Calculate the pH of a solution that is 0.205M benzoic acid and 0.230M sodium benzoate, a salt whose anion is the conjugate base of benzoic acid. (The pKa of benzoic acid
Answers
Answer:
pH = 4.25
Explanation:
A solution composed of a weak acid and its conjugate base is a buffer solution. To calculate the pH of a buffer solution we use the Henderson-Hasselbach equation:
pH = pKa + log ([conjugate base]/[weak acid]
In this case, we have the following data:
[conjugate base] = [sodium benzoate] = 0.230 M
[weak acid] = [benzoic acid] = 0.205 M
The pKa of benzoic acid is 4.2. So, we introduce the data in the equation:
pH = 4.2 + log (0.230 M/0.205 M) = 4.2 + 0.050 = 4.25
take it before its gone
Answers
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
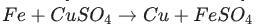
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
