why would a percent yield be less than 100%? give a reason
Answers
Answer:
Usually, percent yield is lower than 100% because the actual yield is often less than the theoretical value. Reasons for this can include incomplete or competing reactions and loss of sample during recovery. ... This can happen when other reactions were occurring that also formed the product.
Explanation:
Related Questions
An atom of 105In has a mass of 104.914558 amu.
mass of1H atom = 1.007825 amu
mass of a neutron = 1.008665 amu
Calculate the mass defect (deficit) in amu/atom.
In atomic #= 49
Answers
The mass defect (deficit) in amu/atom is 0.109879 amu/atom of an atom of 105In has a mass of 104.914558 amu.
The mass of an atom is the sum of the masses of its protons, neutrons, and electrons.
The mass of an atom is generally less than the sum of the masses of its subatomic particles.
The difference between the sum of the masses of its protons, neutrons, and electrons and the mass of an atom is
known as the mass defect.
Mass defect = [(Z × mp + N × mn) − M]
where, Z = the atomic number; N = the number of neutrons; mp = mass of proton; mn = mass of neutron; and M = mass of nucleus.
In this case, mass defect = [(49 × 1.007825 + 56 × 1.008665) − 104.914558] amu/atom = 0.109879 amu/atom.
To learn more about mass defect click here https://brainly.com/question/19828097
#SPJ11
Pls help!
Many thanks

Answers
The shortest covalent bond is the H-F bond. Therefore, option (A) is correct.
What is the bond length order of hydrogen halides?The electronegativity decreases down the group and the Fluorine is the most electronegative atom in the periodic table. The size of the atoms also increases as we go down the group.
The bond length of the hydrogen halides will follow the order HF < HCl < HBr < HI. The radius of the Iodine atom is the largest and to form a molecule, the hydrogen atom will be farthest as compared to all hydrogen halides.
The molecule with Fluorine will have the shortest bond length because its atom has the smallest size, making them attach very closely. So the covalent bond of hydrogen fluoride will be the shortest.
The relationship between bond length and bond strength is inverse in nature. The bond length of HI is the greatest, it will have the least bond strength. So the order for bond strength of the hydrogen halides is HF > HCl > HBr > HI.
Learn more about hydrogen halides, here:
https://brainly.com/question/12115740
#SPJ1
how are crystal structures affected with increasing atomic radius
Answers
Increasing atomic radius can have a significant impact on the crystal structure of a material, affecting parameters such as interatomic distances, coordination numbers, lattice parameters, symmetry, and mechanical properties.
As the atomic radius increases, there are several effects on the crystal structure of a material:
1. Interatomic Distance: With increasing atomic radius, the average distance between neighboring atoms in a crystal lattice also increases. This leads to a larger unit cell and a decrease in atomic packing density.
2. Coordination Number: The coordination number refers to the number of atoms surrounding an atom in a crystal lattice. As the atomic radius increases, the coordination number may change. For example, a smaller atom might have a higher coordination number, while a larger atom might have a lower coordination number. This can influence the overall structure and stability of the crystal.
3. Lattice Parameters: The lattice parameters, such as the lattice constant or unit cell dimensions, may be affected by changes in atomic radius. An increase in atomic radius can result in an expansion of the crystal lattice, leading to changes in the lattice parameters.
4. Symmetry: Changes in atomic radius can affect the symmetry of the crystal structure. Larger atoms may disrupt the symmetry and introduce distortions or vacancies in the crystal lattice.
5. Mechanical Properties: The mechanical properties of a material, such as its strength, hardness, and flexibility, can be influenced by changes in atomic radius. Alterations in atomic size can affect the bonding forces between atoms and, consequently, the material's mechanical behavior.
Learn more about atomic radius at https://brainly.com/question/13126562
#SPJ11
how many grams of caco3 (ksp = 8.7 ×10-9) will dissolve in 230 ml of 0.0500 m ca(no3)2?
Answers
The mass of the calcium carbonate that would dissolve in the compound is 1.15 g.
What is the mass of the compound?We know that the solubility product has to do with the equilibrium constant that we can be able to use in the process of trying to find the amount of the substance that is going to dissolve in the solution.
In this case, we have the volume and the concentration of the salt and we want to find the amount of the calcium carbonate that is going to be dissolved in the solution as we have it in the question.
Thus we can write;
Number of moles = mass/molar mass
Number of moles = concentration * volume
= 230/1000 L * 0.0500 M
= 0.0115 moles
The molar mass of calcium carbonate is 100 g/mol
Mass of the compound = 0.0115 moles * 100 g/mol
= 1.15 g
Learn more about solubility product:https://brainly.com/question/1419865
#SPJ1
what carbonyl compound and alcohol are formed by hydrolysis of each acetal
Answers
Acetals can be hydrolyzed using catalytic acid to produce a carbonyl compound and alcohol. If the acid concentration is increased, acetal can be hydrolyzed back to its initial aldehyde or ketone form.
This mechanism occurs in the opposite direction of the acetal formation mechanism. The hydrolysis of each acetal generates a carbonyl compound and an alcohol.What are Acetals?Acetals are organic compounds that are formed by the reaction of an aldehyde or ketone with two molecules of alcohol, and they have the following general structure: R1R2C(OR')2.Acetals can be regarded as derived from hemiacetals, which are formed by the reaction of an aldehyde or ketone with one molecule of alcohol.The carbonyl carbon in an acetal is bonded to two alkoxide (OR) groups, while the carbonyl carbon in a hemiacetal is bonded to only one. As a result, acetals are more stable than hemiacetals. Acetals are widely used in organic synthesis, including as protecting groups for carbonyl groups in reactions that would otherwise destroy them.Example:Acetal hydrolysis occurs when an acid catalyst is used to cleave the two ether bonds in the molecule. When an acetal is hydrolyzed with an acid catalyst such as H2SO4, a carbonyl compound and an alcohol are formed.Example:H2SO4 is added to the acetal, which hydrolyzes it, producing an aldehyde or ketone and two alcohol molecules. For example, if dimethyl acetal is hydrolyzed, it will yield acetone and two methanol molecules.
To know more about molecules , visit ;
https://brainly.com/question/475709
#SPJ11
describe the simalarities and diffrences between the isotopes 18 o 8 and 16 o 8
Answers
Same number of protons and different number of neutrons.
Oxygen is a chemical element with 8 protons. It is capable of achieving a noble gas electronic configuration by accepting two electrons. Oxygen is important for many living organisms.
Oxygen-16 and oxygen-18 differ in the number of neutrons in their nuclei. Oxygen-16 has 8 neutrons, while oxygen-18 has 10 neutrons. Oxygen-16 and oxygen-18 have the same number of protons and electrons. Both neutrons and protons have mass, so changing the number of neutrons changes the mass of oxygen. These elements with different numbers of neutrons are referred to as isotopes. Both oxygen-16 and oxygen-18 are stable isotopes of oxygen and are not radioactive.
To know more about the Oxygen, here
brainly.com/question/2272415
#SPJ4
what is a simple explanation of electrolysis??? :)
Answers
Answer:
electrolysis is the process by which electric current is passed through a substance to effect a chemical change.
Answer:
The production of a chemical reaction by passing an electric current through an electrolyte is called electrolysis. We know that an electrolyte contains ions, which are charged. The positively charged ions are called cations, because they are attracted to the cathode, and the negatively charged ones are called anions because they are attracted to the anode. We know that unlike charges attract and like charges repel. Cations, being positively charged, get attracted to the negatively charged cathode and move toward it. Anions, being negatively charged, get attracted to the positively charged anode and move toward it. This explains how ions move in an electrolytic cell, and thus ‘conduct’ an electric current. A chemical reaction takes place at the anode and the cathode. This can be observed as the formation of bubbles (due to the production of gases) or deposition of metal on the electrodes or a change in the color of the electrolyte. The reaction varies depending on the metals used for the electrodes and the electrolyte chosen. Electrolysis of a solution of sodium chloride (NaCl) produces hydrogen gas (H2), chlorine gas (Cl2), and sodium hydroxide (NaOFI).
The pictures of electrolysis examples are shown below:


If times going on forever when did it begin
Answers
Answer:
it has always begain it has never stopped therefore it never begain but it will never end as well hope this doesn't confuse you
Explanation:
3.4 x 1023 atoms of Na in moles
Answers
The number of moles of sodium (Na) in 3.4 x 10^23 atoms is approximately 5.64 moles.
In the first paragraph, the main answer is that there are approximately 5.64 moles of sodium (Na) in 3.4 x 10^23 atoms.
Now, let's explain the calculation in the second paragraph. The mole is a unit of measurement used in chemistry to quantify the amount of a substance. One mole of any element contains Avogadro's number of atoms, which is approximately 6.022 x 10^23. In this case, we have 3.4 x 10^23 atoms of sodium (Na). To convert this into moles, we divide the number of atoms by Avogadro's number.
Mathematically, the calculation is as follows:
Moles of Na = (Number of atoms of Na) / (Avogadro's number)
Moles of Na = (3.4 x 10^23) / (6.022 x 10^23)
Moles of Na ≈ 5.64 moles
Therefore, there are approximately 5.64 moles of sodium (Na) in 3.4 x 10^23 atoms.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
which of these three quantities is proportional to concentration? absorbance molar absorptivity transmittance
Answers
Absorbance is the quantity that is proportional to concentration among the three options provided, which also include molar absorptivity and transmittance.
According to the Beer-Lambert Law, absorbance (A) is directly proportional to the concentration (c) of a solution. The relationship can be expressed as A = εcl, where ε is the molar absorptivity (a constant for a particular substance), c is the concentration of the solution, and l is the path length of light through the solution. As concentration increases, absorbance also increases, indicating that more light is being absorbed by the solution.
Molar absorptivity, on the other hand, is a constant that depends on the substance being measured and the wavelength of light used. It indicates how well a substance absorbs light at a particular wavelength and does not vary with concentration.
Transmittance (T) is the fraction of light that passes through a solution without being absorbed. It is related to absorbance, but not directly proportional to concentration. As the concentration of a solution increases, transmittance usually decreases due to increased light absorption.
In summary, absorbance is the quantity that is proportional to concentration among the three options. Molar absorptivity is a constant property of a substance, and transmittance is related to absorbance but not directly proportional to concentration.
To know more about absorbance, refer to the link below:
https://brainly.com/question/13492847#
#SPJ11
The mechanism for the reaction 2H₂O₂ (aq) → 2H₂O(aq) + O₂(g) in the presence of: I (aq) is proposed to be
Step 1: H₂O₂ (aq) + I (aq) → H₂O(aq) + OI (aq) (slow)
Step 2: H₂O₂ (aq) + OI (aq) → H₂O(aq) + O₂(g)+ I (aq) (fast)
1-Write the equation for the overall reaction:
2-Identify the intermediate:
3-What is the molecularity of the rate-determining step?
Answers
2H2O2 (aq) 2H2O(aq) + O2 is the overall reaction (g)
OI- Intermediate
The molecularity of the RDS is 2
What is mecahnism?A reaction mechanism has to do with the way that the sequence of elementary reactions are taking place as we can see here.
The rate of a reaction, the energy changes that take place throughout the reaction, and the variables that affect the reaction can all be learned a lot from the reaction mechanisms. Additionally, they can serve as a guide for creating more effective and focused chemical processes as well as an aid in explaining the behavior of a chemical reaction as it is observed.
Visit to learn more about mecahnims:https://brainly.com/question/30698052
#SPJ1
Calculate the volume in L of 11.6 moles of Neon at 120 K when it has a pressure of 25.9 atm
Answers
Answer:
The volume of the gas is approximately 4.41 liters
Explanation:
The details of the data of the Neon gas are;
The number of moles of Neon gas present, n = 11.6 moles
The temperature of the sample of Neon gas, T = 120 K
The pressure of the sample of the Neon gas, P = 25.6 atm
By the ideal gas equation, we have;
P·V = n·R·T
Where;
R = The universal gal constant = 0.08205 L·atm·mol⁻¹·K⁻¹
Therefore, we get;
V = n·R·T/P
Which gives;
V = 11.6 moles × 0.08205 L·atm·mol⁻¹·K⁻¹ × 120 K/(25.9 atm) ≈ 4.4097915 L
The volume of the gas, V ≈ 4.41 L.
what is the balance of S8+Br2=S3Br7
Answers
Answer:
3S₈ + 28Br₂ => 8S₃Br₇
Explanation:
Start with either sulfur (S) or bromine (Br) and balance ...
3S₈ + Br₂ => 8S₃Br₇ or S₈ + 7/2Br₂ => S₃Br₇
Balance the remaining reactant ...
3S₈ + 56/2Br₂ => 8S₃Br₇
Remove fractions by multiplying by the fraction's denominator
2(3S₈ + 56/2Br₂ => 8S₃Br₇) => 6S₈ + 56Br₂ => 16S₃Br₇
Reduce to smallest whole number ratio => standard equation at STP ...
3S₈ + 28Br₂ => 8S₃Br₇
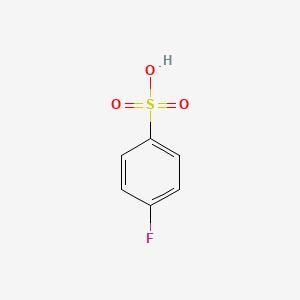
p‑fluoroanisole reacts with sulfur trioxide and sulfuric acid. draw the major product of this substitution reaction; if applicable, minimize formal charges via expanded octets. assume 1 equivalent of reagents is used.
Answers
The major product of the reaction between p‑fluoroanisole and sulfur trioxide and sulfuric acid is p‑fluorobenzene sulfonic acid. The sulfonic acid group (-SO3H) substitutes for the methoxy group (-OCH3) on the benzene ring. The product is shown below:
F
|
H3C--O--C6H4--SO3H
|
H
Note that the sulfur atom has an expanded octet, with 10 electrons in its valence shell.
One of the most well-known instances of an electrophilic aromatic substitution reaction is the reaction of p-fluoroanisole with sulphur trioxide and sulfuric acid. The electrophile in this reaction is a highly reactive sulphur trioxide-sulfuric acid complex, and the sulphur trioxide and sulfuric acid serve as its sources. The electrophile assaults the p-electron-rich fluoroanisole's aromatic ring, removing the methoxy group and replacing it with a sulfonic acid (-SO3H) group.
P-fluorobenzenesulfonic acid is a helpful intermediate in many chemical synthesis reactions due to the sulfonic acid group's high acidity and polarity. Additionally, a variety of chemical processes, including esterification, amidation, and reduction, can be used to further modify the sulfonic acid group in order to produce other derivatives.
The sulfonic acid group's sulphur atom has an extended octet, which implies it contains more than eight valence electrons in its outer shell. For lesser elements like carbon, nitrogen, and oxygen, this is unusual, but for heavier elements like sulphur and phosphorus, it is rather typical. Expanded octets are typically seen when the central atom can interact with empty d orbitals that can form bonds.
For more question on substitution reaction click on
https://brainly.com/question/27906533
#SPJ4
gamma rays have an approximate wavelength of 1.0x10^-13 meters. what is the approximate energy of a gamma-ray?
Answers
Answer:
E = 19.89 × 10⁻¹³ J
Explanation:
Given data:
Wavelength of gamma ray= 1.0× 10⁻¹³ m
Energy of gamma ray = ?
Solution:
Formula:
E = h c/λ
h = plancks constant = 6.63× 10⁻³⁴ js
c = speed of light = 3 ×10⁸ m/s
by putting values,
E = 6.63× 10⁻³⁴ js × 3 ×10⁸ m/s /1.0× 10⁻¹³ m
E = 19.89 × 10⁻²⁶ J.m/ 1.0× 10⁻¹³ m
E = 19.89 × 10⁻¹³ J
Activity 2: Who's My Family? A fire has occurred in a nearby maternity clinic. The assigned nurse quickly rushed out of the place to secure the newly born babies. Unfortunately, there were some babies without their identification bracelets. Using your knowledge about codominance inheritance will help bring these babies back to their correct parents.
Answers
Codominance is a type of inheritance pattern in which both alleles of a gene are expressed equally in the phenotype of the individual. This means that if a baby inherits two different alleles for a particular trait, both will be expressed in the baby's physical appearance.
In the case of the missing identification bracelets, the nurse could use the principle of codominance to help identify the babies and return them to their correct parents. For example, if one baby has a parent with blood type A and the other has a parent with blood type B, and both babies have blood type AB due to codominance, then the nurse could match the babies with their correct parents based on their blood type.
Similarly, if there are other observable traits that exhibit codominance, such as eye color or skin tone, the nurse could use these to help identify the babies and return them to their correct parents. By understanding and applying the principles of codominance inheritance, the nurse could help ensure that each baby is reunited with their rightful family.
To learn more about inheritance refer to:
brainly.com/question/14930526
#SPJ4
Predict the decreasing order of vapor pressure for the following compounds i) CH3CH₂OH; ii) CH3CH₂F; iii) CH3CH3 a) i>ii >iii b)i>iii>ii c)ii> i>iii d) ii > iii >i e) iii > ii >i [(CH₂)₂COL with those
Answers
\(CH_3CH_2OH\) is a polar compound and has hydrogen bonding. It has a higher boiling point. It will have the lowest vapor pressure among the three compounds.
Vapor pressure is the pressure produced by the vapors of a liquid in a sealed container when the liquid is heated. It is a property of a liquid that describes the tendency of a liquid to evaporate. It is measured in units of pressure, typically in mmHg or torr. The higher the vapor pressure, the more volatile a compound is. So, the decreasing order of vapor pressure for the given compounds is: \(CH_3CH_3 > CH_3CH_2F > CH_3CH_2OH\)
Here, \(CH_3CH_3\) is a non-polar compound while \(CH_3CH_2F\) and \(CH_3CH_2OH\) are polar compounds. The polar compound has hydrogen bonding, which increases the intermolecular forces between molecules. As a result, the polar compound will have a lower vapor pressure than the non-polar compound.
To learn more about vapor pressure click here https://brainly.com/question/25715932
#SPJ11
c2h5nh2 molecular or ionic
Answers
C₂H₅NH₂ is a covalent molecule and not ionic. It is a weak organic base. Sparingly soluble or insoluble in water.
What is ethylamine?Ethylamine is an organic compound classified under amines with the functional group of NH₂. The formula of ethylamine is C₂H₅NH₂. The compound is a weak base.
Ethylamine is formed by the covalent bonding between the carbon in the ethyl group and nitrogen from the amino group. It is an aliphatic amine and thus weaker base than cyclic and aromatic amines. Amines are generally sparingly soluble in water.
Therefore, ethylamine, C₂H₅NH₂ is not an ionic molecule and the formula represents it molecular composition.
To find more on ethylamine, refer here:
https://brainly.com/question/9439418
#SPJ1
which label signal word indicates a pesticide that is least hazardous to humans
Answers
The label signal word that indicates a pesticide is least hazardous to humans is "Caution."
Pesticides are labeled with signal words to indicate the level of potential harm they pose to humans and the environment. The four signal words in decreasing order of hazard are Danger, Warning, Caution, and No signal word. The signal word "Caution" indicates that the pesticide is relatively low in toxicity and poses a minimal risk of harmful effects to humans.
However, it is important to note that even pesticides labeled with "Caution" should be used with care and according to the label instructions to prevent any unintended exposure or harm.
It is also essential to follow all safety precautions when using any pesticide and to store and dispose of it properly to avoid any negative effects on humans, animals, and the environment.
For more questions like Pesticide click the link below:
https://brainly.com/question/31600813
#SPJ11
a container has 0.553 mol of a gas and a volume of 253 ml. what is the volume if 0.365 mol of the gas is added to the container?
Answers
Assuming the temperature and pressure are constant, we can use the ideal gas law to solve for the new volume of the container:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
Since the temperature and pressure are constant, we can write:
(P1)(V1) = n1RT and (P2)(V2) = n2RT
where the subscripts 1 and 2 refer to the initial and final states, respectively.
Solving for V2, we get:
V2 = (n2/n1) × (V1)
where V1 is the initial volume, n1 is the initial number of moles, and n2 is the final number of moles.
Substituting the given values, we get:
V2 = (0.918 mol / 0.553 mol) × (253 ml) ≈ 420 ml
Therefore, the volume of the container with the additional gas is approximately 420 ml
To know more about refer ideal gas law here
brainly.com/question//30458409#
#SPJ11
Which of the following equation is balanced?

Answers
Answer:
2fe2o3+3c=4fe+3co2
Explanation:
because 2×2 =4 fe &2×3=6 so we put it like 3co2
Use the molar solubility 3.27 * 10-11 M in pure water to calculate Ksp for NiS
Answers
This value indicates that NiS is very slightly soluble in water and thus will not readily dissolve in it.
What is soluble?Soluble describes substances that can be dissolved in a liquid. When a substance is soluble, it is able to form a homogenous solution with the liquid, meaning that the substance will spread out evenly throughout the liquid. Common examples of soluble substances include sugar and salt, which can be dissolved in water. Other substances, such as oil, are insoluble and will not dissolve in a liquid.
The Ksp of NiS is equal to the molar solubility of NiS in pure water raised to the power of two. This is because NiS dissociates into two ions in water (Ni2+ and S2-). Therefore, the Ksp of NiS can be calculated as follows:
Ksp = (3.27 x 10-11 M)2 = 1.06 x 10-21
This means that the solubility product constant (Ksp) of NiS is equal to 1.06 x 10-21. This value indicates that NiS is very slightly soluble in water and thus will not readily dissolve in it.
To learn more about soluble
https://brainly.com/question/1593513
#SPJ4
HURRRRRRY PLEASEEEE HELP!!!!!!
What type of plate boundary are the arrows on the image showing?

Answers
Answer:
Transform boundaries because they're sliding past each other.
I hope this helped!
Elemental potassium reacts violently with water. The reactionusually results in a lilac-colored flame, fueled by the formation offlammable hydrogen gas, as seen in the reaction below. How manygrams of hydrogen are produced when 43.7 grams of potassiumare tossed into water? Show all work and give your answers tothree significant figures.2K + 2H2O -> 2KOH + H2M(K) = 39.10 g/mol; M(H2O) = 18.01 g/mol; M(KOH) = 56.11 g/mol;M(H2) = 2.02 g/mol
Answers
Explanation:
First, we need to rewrite the equation:
2K + 2H2O -> 2KOH + H2
This equation tells us that 2 moles of K react with 2 moles of H2O and produce 2 moles of KOH and 1 mole of H2.
If we have 43.7 grams of potassium, we can transform it into moles, compare with the ratio between Potassium and Hydrogen (H2) and then, calculate the quantity of hydrogen in grams.
So first, let's transform 43.7 grams of K into moles using the following formula:
moles = mass/molar mass
molar mass of K = 39.10 g/mol
moles = 43.7/39.10
moles = 1.12 moles
So:
2 moles of K --- 1 moles of H2
1.12 moles of K --- x moles of H2
2x = 1.12
x = 1.12/2
x = 0.559 moles of H2
Now let's transform moles into grams using the following formula:
mass = moles x molar mass
molar mass of H2 = 2.02 g/mol
mass = 0.559 x 2.02
mass = 1.13 g
Answer: It is produced 1.13 grams of H2.
HEY YALL PLS HELP ME ANSWER THIS QUESTION- THE CORRECT ANSWER SHALL RECEIVE 10 POINTS xx
~What technique is used to separate water from salt water‽
thanks yall xx )
Answers
Answer:
Simple distillation is a method for separating the solvent from a solution.
Explanation:
For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates.
Answer:
Stealing the last of your points on questions, it's been fun and I will most likely do it more in the future but for now this is the end. Thank you for the many points friend.
Explanation:
⏁⊑⏃⋏☍ ⊬⍜⎍ ⎎⍜⍀ ⏁⊑⟒ ⌿⍜⟟⋏⏁⌇ ⎅⟒⏃⍀ ⎎⍀⟟⟒⋏⎅, ⟟ ⏃⋔ ⎎⍜⍀⟒⎐⟒⍀ ⟟⋏ ⊬⍜⎍⍀ ⎅⟒⏚⏁.
T/F: All four jovian planets are made primarily of hydrogen and oxygen
Answers
False. While all four jovian planets (Jupiter, Saturn, Uranus, and Neptune) are primarily made of gas, they are not primarily made of hydrogen and oxygen.
Jupiter and Saturn are primarily made of hydrogen and helium, while Uranus and Neptune are primarily made of ices (water, methane, and ammonia) and rock. The exact composition of these planets varies based on their distance from the sun, the temperature of their interiors, and other factors. However, it is generally accepted that the jovian planets are mostly made of gases and ices, with only a small solid core at their center.
False. All four Jovian planets (Jupiter, Saturn, Uranus, and Neptune) are primarily composed of hydrogen and helium, not hydrogen and oxygen. These gas giants have a small rocky core, surrounded by a thick layer of gas, mostly hydrogen and helium. The composition and size of their cores vary, but the primary elements remain consistent throughout. While some water, ammonia, and methane are present in their atmospheres, oxygen is not a dominant component in their overall composition.
To know about planets :
https://brainly.com/question/29765555
#SPJ11
Which statement is the best description of a chemical reaction? A.The combustion of a substance B.The decomposition of particles C.Mixing two substances D.Forming or breaking bonds
Answers
The answer would be D. Forming or breaking bonds.
When forming or breaking bonds, a new substance is created and it’s no longer just a physical change. Sometimes when bonds are breaking and new ones are forming there can be a change in composition.
Hope this helps !
To solve this we must know the concept of chemical reaction. The correct option is option D that is forming or breaking of bonds. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved.
Chemical reaction is a reaction in which the breaking of old bond from the reactant take place while on the product side, formation of new bond take place to form product.
Therefore, the correct option is option D that is Forming or breaking of bonds take place during chemical reaction.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ5
What is an element?
Question 1 options:
A. Material that cannot be broken down any further
B. The sum of protons and neutrons in an atom
C. A region of tightly packed protons
D. Negatively charged ions
Answers
An element is the sum of protons and neutrons in an atom
What is an element ?An element is a fundamental thing that is difficult to divide into smaller parts. A substance that cannot be broken down by non-nuclear reactions is referred to as an element in chemistry and physics. An element is a unique component of a bigger system or set in computing and mathematics.
For instance, whereas water (H2O), which is composed of hydrogen and oxygen, is an element, hydrogen and oxygen are not elements.The Periodic Table is divided into three main categories metals, nonmetals, and metalloids. Each group's elements share a number of physical and chemical characteristics.Learn more about Elements here:
https://brainly.com/question/6258301
#SPJ4
please help I tired of doing this
Select the correct answer.
What additional evidence did Alfred Wegener provide to justify his theory of continental drill
A presence of similar geological features on most of the continents
B presence of coal deposits in the South Polar Region
C proof of periodic reversals in Earth's polarity
D. presence of the mid-ocean ridge
Answers
Answer:
uhh
Explanation:
Wegener found similar evidence when he discovered tropical plant fossils in the frozen region of the Arctic Circle. As Wegener collected more data, he realized the explanation that best fit all the climate, rock, and fossil observations involved moving continents.