why dont we just transfer electron directly from nadh to o2
Answers
The transfer of electrons from NADH to oxygen is a crucial step in aerobic respiration, which is the process by which cells produce energy. However, the transfer of electrons directly from NADH to oxygen is not possible due to the high energy barrier that exists between these two molecules.
Instead, electrons are transferred from NADH to the electron transport chain, a series of electron carriers embedded in the inner mitochondrial membrane. The electron transport chain uses the energy from the electrons to pump protons out of the mitochondrial matrix, creating an electrochemical gradient that is used to drive ATP synthesis. Oxygen serves as the final electron acceptor in the electron transport chain, forming water as a byproduct. In summary, while it would be more efficient to transfer electrons directly from NADH to oxygen, the electron transport chain is necessary to overcome the energy barrier and produce ATP through oxidative phosphorylation.
The reason we don't directly transfer electrons from NADH to O2 is because it would release a large amount of energy all at once, which could be harmful to cells. Instead, the electron transport chain (ETC) in cellular respiration gradually transfers the electrons from NADH to O2 through a series of protein complexes. This controlled transfer allows cells to harness the energy released in the form of a proton gradient, which is then used to generate ATP through oxidative phosphorylation. By not directly transferring electrons, cells can efficiently capture and utilize the energy from NADH in a safe and controlled manner.
For more information on energy barrier visit:
brainly.com/question/18880410
#SPJ11
Related Questions
Protons: 8
Neutrons: 9
Electrons: 10

Answers
Answer:
Name Fluorine
Atomic Mass 18.998 atomic mass units
Number of Protons 9
Number of Neutrons 10
Number of Electrons 9
Explanation:
Will give brainliest if you answer quickly and correctly :)
An atom X has 5 energy levels of electrons outside its nucleus and 74 neutrons inside its nucleus. Identify this atom
a. P
b. Ca
c. I
d. Cd
e. Fr
Answers
Iodine has 53 protons and 74 neutrons
Answer:
C. I (iodine)
Explanation:
it would be Iodine because it has 5 energy levels of electrons and has a total of 74 neutrons inside its nucleus!
Hope this helps, have a good day!
What is a trial? please
Answers
What mass of NO2 can be produced when 678 mL of oxygen at STP and 256 grams of NH3 are mixed together?
Answers
Answer:
WHT55
Explanation:
i experianced this before
Select the correct answer.
A car moves with an average speed of 45 miles/hour. How long does the car take to travel 90 miles?
Group of answer choices
Answers
The enthalpy of vaporization of Subtance X i 17. 0kJmol and it normal boiling point i 90. °C. Calculate the vapor preure of X at −92. °C. Round your anwer to 2 ignificant digit
Answers
At −92°C, the vapour pressure of the substance X is 5.5 atm
According to Clausius Clapeyron equation,
ln (P₂/P₁) = -ΔHvap /R (1/T₂ - 1/T₁) -------> (i)
Here,
P₁ = 1 atm
T₁ = 90°C = (90 +273)K = 363K
T₂= -92°C = (-92+ 273)K = 181 K
ΔHvap = 17 KJ/mol
R = 8.314 J/K/mol
To find: P₂
Substituting the above given values in equation (i) we get,
ln (P₂/P₁) = -ΔHvap /R (1/T₂ - 1/T₁)
ln(P₂/ 1 atm)= -17 KJmol⁻¹ / 8.314 JK⁻¹mol⁻¹ ( 1/181K -1/363K)
ln P₂ = -17 KJmol⁻¹ / 0.008314 KJK⁻¹mol⁻¹ ( 0.0028K)
2.303 log (P₂) = - 730240.55 atm
log (P₂) = - 730240.55 atm/ 2.303
log (P₂) = -317082.306 atm
P₂ = antilog (-317082.306)
P₂ = 5.5 atm (upto two significant figures)
Thus, the vapour pressure of X at −92°C is 5.5 atm.
To know more about vapour pressure here
https://brainly.com/question/13213515
#SPJ4
In which layer of the ocean do surface currents flow?
Answers
Answer:
ooh sorry, but will this help you now:
Ocean dynamics define and describe the motion of water within the oceans. Ocean temperature and motion fields can be separated into three distinct layers: mixed (surface) layer, upper ocean (above the thermocline), and deep ocean. Ocean currents are measured in sverdrup (sv), where 1 sv is equivalent to a volume flow rate of 1,000,000 m (35,000,000 cu ft) per second.
Surface currents, which make up only 8% of all water in the ocean, are generally restricted to the upper 4…
Explanation:
Hope this helps :)
what two things can fluids do?
Answers
choose the correct structural formula for the alcohol formed in this reaction
Answers
Answer:
The formula is CnH2n+1OH
Explanation:
Can somebody plz help answer these true or false questions correctly (only the ones you know that are right)
Thanks :3
WILL MARK BRAINLIEST WHOEVER ANSWERS FIRST..promise! :DDDD

Answers
Explanation:
1.False
2.False
3.True
4.True
5.False
In the coordination compound [Cr(NH3)2(en)Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively, are
Answers
The coordination number (C.N.) is 6 and oxidation number (O.N.) is +4 of the metal atom.
C.N. = 6; O.N. = +4
What is Co-ordination number ?The coordination number, also known as ligancy, of a central atom in a molecule or crystal in chemistry, crystallography, and materials science refers to the quantity of atoms, molecules, or ions bound to it. A ligand is the ion, molecule, or atom that surrounds the center ion, molecule, or atom.The number of atoms, ions, or molecules that a central atom or ion in a complex, coordination compound, or crystal holds as its closest neighbors.What is Oxidation number ?The total number of electrons that an atom acquires or loses to establish a chemical connection with another atom is known as the oxidation number, also known as the oxidation state.Each atom involved in an oxidation-reduction reaction has an oxidation number that represents how many electrons it may take, give, or share.To view more questions of coordination number, refer to:
https://brainly.com/question/8717978?referrer=searchResults
#SPJ4
2Al(s) + 6HCl(aq) ––––> 2AlCl3(aq) + 3H2(g) According to the equation above, how many grams of aluminum metal are needed to completely react with 3.83 mol of hydrochloric acid? A) 310 g B) 46.6 g C) 34.4 g D) 3.83 g E) 103.3 g
Answers
The mass of aluminum needed to completely react with 3.83 mol of hydrochloric acid is approximately 34.44 grams.
To determine the mass of aluminum needed to react with 3.83 mol of hydrochloric acid, we need to use the stoichiometry of the balanced equation.
From the balanced equation: 2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
We can see that the mole ratio between aluminum (Al) and hydrochloric acid (HCl) is 2:6, or simplified, 1:3. This means that for every 1 mole of aluminum, we need 3 moles of hydrochloric acid.
Given that we have 3.83 mol of hydrochloric acid, we can set up the following proportion:
1 mol Al / 3 mol HCl = x mol Al / 3.83 mol HCl
Simplifying the proportion, we find:
x = (1 mol Al / 3 mol HCl) * 3.83 mol HCl
x = 1.277 mol Al
Now, we need to calculate the mass of aluminum using its molar mass. The molar mass of aluminum is approximately 26.98 g/mol.
Mass of aluminum = 1.277 mol Al * 26.98 g/mol Al
Mass of aluminum = 34.44 g
Know more about hydrochloric acid here:
https://brainly.com/question/24784580
#SPJ11
PLEASE LOOK AT IMAGINE SCIENCE

Answers
Answer: C
Explanation: because Pangaea broke apart and made smaller continents.
The total number of atoms represented by the formula (NH4)2Cr2O7 is
a. 23
b. 11
c. 19
d. 18
will give branliest for quick and correct answers
Answers
Answer:
its 19 I'm pretty sure about
Answer:
c is correct and my girl friend
What is the atomic mass of a hypothetical element that consists of the following isotopes in the indicated natural abundances?
1)
i)Isotope Isotopic mass (amu) Relative abundance %
1 78.9 10.4
2 82.9 15.0
3 83.9 74.6
Express your answer numerically in atomic mass units.
ii) What is the atomic mass of the element that consists of the following isotopes in the indicated natural abundances?
Isotope Isotopic mass (amu) Relative abundance (%)
1 57.93 67.76
2 59.93 26.16
3 60.93 1.25
4 61.93 3.66
5 63.93 1.16
Express the mass numerically in atomic mass units.
iii) Naturally occurring iodine has an
atomic mass of 126.9045. A 12.3849-g sample
of iodine is accidentally
contaminated with 1.00070 g of ^129I, a
synthetic radioisotope of iodine used in the treatment
of certain diseases of the thyroid gland.
The mass of ^129 I is 128.9050 .
Find the apparent "atomic mass" of the contaminated iodine.
Express your answer using six significant figures.
Atomic Mass = ? amu
Answers
i) Atomic mass = 83.117 amu.
ii) Atomic mass = 58.679207 amu.
iii) Apparent atomic mass = 126.943682 amu.
i) For the first hypothetical element, we are given three isotopes with their respective isotopic masses and natural abundances. To calculate the atomic mass, we multiply the mass of each isotope by its relative abundance, and then sum up the results. Using the provided values:
Atomic mass = (78.9 amu × 10.4%) + (82.9 amu × 15.0%) + (83.9 amu × 74.6%)
= 8.2016 amu + 12.435 amu + 62.4814 amu
= 83.117 amu
Therefore, the atomic mass of the hypothetical element is approximately 83.117 amu.
ii) Similarly, for the second hypothetical element, we have five isotopes with their isotopic masses and natural abundances. Using the same formula:
Atomic mass = (57.93 amu × 67.76%) + (59.93 amu × 26.16%) + (60.93 amu × 1.25%) + (61.93 amu × 3.66%) + (63.93 amu × 1.16%)
= 39.235368 amu + 15.675888 amu + 0.761625 amu + 2.265438 amu + 0.741888 amu
= 58.679207 amu
Therefore, the atomic mass of the second hypothetical element is approximately 58.679207 amu.
iii) To find the apparent atomic mass of the contaminated iodine, we consider the total mass of the sample, which includes the mass of naturally occurring iodine and the synthetic radioisotope. The apparent atomic mass is calculated by multiplying the mass of each component by its respective atomic mass, and then dividing by the total mass. Using the given values:
Apparent atomic mass = [(12.3849 g × 126.9045 amu) + (1.00070 g × 128.9050 amu)] / (12.3849 g + 1.00070 g)
= (1568.47174005 amu + 128.9322205 amu) / 13.3856 g
= 1697.40396055 amu / 13.3856 g
= 126.943682 amu
Therefore, the apparent atomic mass of the contaminated iodine is approximately 126.943682 amu.
To learn more about atomic mass click here brainly.com/question/30678413
#SPJ11
Explain ow accommodating and co
llaborating might resolve conflict and contribute to harmonious relationships during your grade 12 academic year
Answers
Accommodating involves one party sacrificing their own interests to satisfy the other party's needs while collaborating involves both parties working together to find a mutually beneficial solution.
Conflicts can be quickly resolved and positive relationships between the parties involved by being accommodating. For instance, one student might decide to abandon their idea in favor of the other student's idea if two students in a group project have opposing opinions on how to approach a task. This can help the group get along better and avoid conflicts.
On the other hand, working together can result in creative answers that benefit both parties. When two people work together, they combine their distinctive perspectives and ideas, which can result in innovative solutions that neither party would have thought of on their own. For instance, if two students disagree on how to complete a group assignment, they can work together and combine their ideas to come up with a more thorough and workable solution.
Learn more about Accommodating
brainly.com/question/30492472
#SPJ4
You mix the sample discussed in the previous two questions with another sample, which you know to be pure acetylsalicylic acid. You grind the mixture thoroughly with a mortar and pestle, and do a melting point determination on the mixed product. You now observe melting over a range of 127 to 133 C. Now what conclusions can you reach regarding the original product
Answers
Answer:
Having high melting point.
Explanation:
The original product has high melting point as compared to the mixture product because in the original product, the element is present in its pure state and we know that pure substances have high melting point as compared to mixture substances due to the presence of strong intercellular forces between them which is hard to break so that's why we can say that the original product has high melting point.
2Hg + O2
What’s the reactants and the products
Answers
Answer:
reactants are mercury and oxygen.the product is mercurricoxide.
a chemist wants to mix a 70% saline solution with a 8 liters of a 25% saline solution to create a solution with 40% salt. how many liters of the 70% solution does she need? (saline is a mixture of salt and water)
Answers
4 liters of the 70% solution are required by the chemist.
briefly:-8 liters of saline solution divided by 25%
Saline solution at 25% = 0.25 x 8 = 2 liters
Let there be x liters of saline solution at 70%.
Salt = 8 plus x
40% of salt is equal to 25% of saline solution plus 70% of saline solution.
2 + (70/100)x = (40/100)(8 + x)
2 + 0.7x = 0.4(8 + x)
2 + 0.7x = 3.2 + 0.4x
assemble similar terms0.7x - 0.4x = 3.2 - 20.3
x = 1.2
x = 1.2 / 0.3
x = 4
4 liters of the 70% solution are required by the chemist.
How can the volume of a dilution be determined?The following equation can be used to determine the volume or concentration of a concentrated or diluted solution: M1V1 = M2V2.
To know more about saline solution visit:-
https://brainly.com/question/29342861
#SPJ4
How do you respond to a chemical exposure to the eye?
Answers
Do these things right away when a chemical splashes on your eye.Use water to irrigate your eye.For the at least 20 minutes, use pristine, lukewarm tap water.Use soap and water to wash your hands.To ensure that no chemicals or soap are still on your hands, thoroughly rinse them.Remove your contacts.
How might chemical spills be avoided?Safety Glasses, goggles, and shields — These products shield the face and eyes from chemical spills.Additionally, they protect them against gases, vapors, and dust.Skin Protection - Clothing items like gloves, boots, & coveralls should be used in order to protect the skin.
A chemical splash accident is what kind of mishap?Chemical splashes are incidents where dangerous substances unintentionally spill, project, aerosolize, or otherwise disseminate inside a laboratory setting.
To know more about chemical exposure visit:
https://brainly.com/question/29629890
#SPJ4
Unit Test Review
Active
1
2
3
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to
another?
O The volume and the shape stay the same.
O The volume increases to fill the new container, but the shape stays the same.
O The volume stays the same, but the shape changes to fit the new container
O The volume and the shape change to fill the new container
Answers
Answer:
the volume stays the same, but the shape changes to fit the new container
Explanation:
because the same amount/volume of liquid is being placed in a new container, the new container has a different shape to the old, thus changing the liquids shape but not volume.
how many glyceraldehyde 3-phosphate (g3p) molecules would be produced by 18 turns of the calvin cycle?
Answers
Eighteen turns of the Calvin cycle would produce 36 G3P molecules.
The Calvin cycle, also known as the dark cycle, is a metabolic process that occurs in plants and algae. The cycle is made up of a series of chemical reactions that convert carbon dioxide into glucose.
Glyceraldehyde 3-phosphate (G3P) is a three-carbon sugar that is one of the products of the Calvin cycle. Six CO2 molecules and six ribulose-1,5-bisphosphate molecules enter the cycle to create twelve 3-phosphoglycerate molecules.
Twelve ATP molecules and twelve NADPH molecules are then used to transform the 3-phosphoglycerate molecules into twelve G3P molecules. Ten out of twelve G3P molecules are used to regenerate six ribulose-1,5-bisphosphate molecules, while two are used to create glucose or other organic compounds.
Each turn of the Calvin cycle produces one G3P molecule, while each glucose molecule requires two G3P molecules. This implies that 36 G3P molecules would be produced by 18 turns of the Calvin cycle.
To know more about Calvin cycle, refer here:
https://brainly.com/question/30808737#
#SPJ11
You have two pieces of jewelry. One of them is supposed to be pure silver. The first piece has a mass of
20.0 g and the other piece has a mass of 44.0 g. Both pieces displace 4.0 mL of water. Silver has a density
of 10.5 g/cm³. Which piece is actually silver?
Answers
The silver piece with mass of 44.0 g is an actual silver.
What is mass?A mass can be defined as a dimensionless quantity representing the amount of matter in a particle or object. The SI unit of mass is kg.
A density is defined as the measurement of how tightly a material is packed together.
To calculate the purity of silver first we have to calculate density of both the silver.
4.0 ml = 4.0 cm3
Density of 20.0 g silver = 20.0g/4.0 cm3 = 5.0 g/ cm3
Density of 44.0 g silver = 44.0g/4.0 cm3 = 11.0 g/ cm3
Thus, the density of 44.0 g of silver is near to density of pure silver hence, 44.0 g of silver is a pure silver.
To learn more about mass, refer to the link below:
https://brainly.com/question/19694949
#SPJ1
Wenner four poles equal method is used to measure the soil resistivity near a 66/11 kV substation using a AEMC 6472 Ground Tester. The readings are recorded at 1, 2, 3, 4 and 5 m intervals of the probe distance. The corresponding soil resistance were measured to be 16.4, 5.29, 3.05, 1.96 and 1.36 2, respectively. Calculate the average soil resistivity in that substation.
Answers
The average soil resistivity near the substation is approximately 5.612 Ω·m.
To calculate the average soil resistivity near the substation, we can use the Wenner four poles equal method and the given soil resistance readings.
The formula for calculating soil resistivity using the Wenner method is:
ρ = (π * spacing * sum of resistance) / (2 * π * probe length)
Where:
ρ = Soil Resistivity
spacing = Distance between the current electrodes (m)
sum of resistance = Sum of the measured soil resistance values (Ω)
probe length = Length of the probe (m)
In this case, the probe distance intervals are 1, 2, 3, 4, and 5 m, and the corresponding soil resistance values are 16.4, 5.29, 3.05, 1.96, and 1.36 Ω, respectively.
Let's calculate the average soil resistivity:
spacing = 1 m (since the distance between the current electrodes is not mentioned, we assume it to be 1 m)
sum of resistance = 16.4 + 5.29 + 3.05 + 1.96 + 1.36 = 28.06 Ω
probe length = 5 m (as given in the intervals)
Using the formula, we have:
ρ = (π * spacing * sum of resistance) / (2 * π * probe length)
= (π * 1 * 28.06) / (2 * π * 5)
= 5.612 Ω·m
Therefore, the average soil resistivity near the substation is approximately 5.612 Ω·m.
Learn more about Wenner method from the given link!
https://brainly.com/question/31715647
#SPJ11
QUICK PLEASE!! 30 PTS! ONLY ANSWER IF YOU'RE SURE!
Describe how rate relationships and activation energy are important in chemical reactions.
Answers
Answer:
The higher the activation energy, the slower the chemical reaction will be.
Explanation:
your welcome
Answer:
The activation energy of a chemical reaction is closely related to its rate. Specifically, the higher the activation energy, the slower the chemical reaction will be. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier.
Explanation: Each and every chemical reaction require some amount of activation energy to begin. The activation energy enables the reactants to move and overcome forces of repulsion. The subsequent collisions between atoms facilitates the breaking of existing bonds and the formation of new ones.
Please help I will give brainly.
Thank you so much
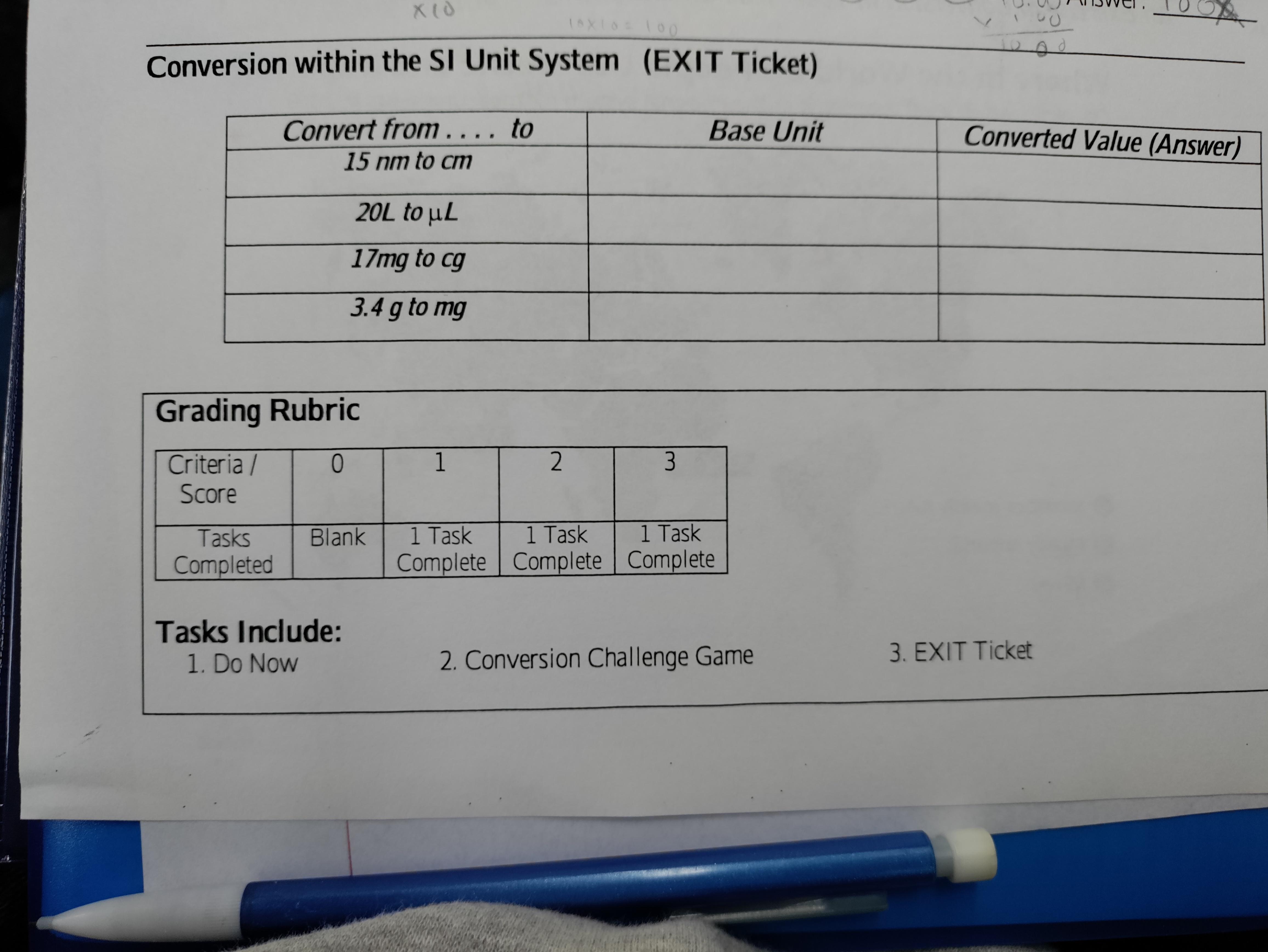
Answers
Answer:
meter. 1.5×10^-6
Litter. 2×10^-5
Kilo Gram. 1.7
Kilo Gram. 3.4 ×10^-3
I hope you understood that and if you don't understand write in comments
what is the molarity of a solution containing 0.325 moles of lactic acid in 250.0 ml of solution?
Answers
To calculate the molarity of the solution, we need to use the formula:
Molarity = moles of solute / liters of solution
Since we are given the moles of lactic acid (0.325) and the volume of solution in milliliters (250.0 ml), we first need to convert the volume to liters by dividing by 1000:
250.0 ml / 1000 = 0.250 L
Now we can substitute the values into the formula:
Molarity = 0.325 moles / 0.250 L
Molarity = 1.30 M
Therefore, the molarity of the solution containing 0.325 moles of lactic acid in 250.0 ml of solution is 1.30 M.
For more question like molarity visit the link below:
https://brainly.com/question/8732513
#SPJ11
What does the difference in macroscopic size of a mole of sugar and a mole of salt suggest about their microscopic representative particles?
Answers
from the human eye i.e from a macroscopic view, a sugar particle is bigger than a salt particle.
from a microscopic view, a mole of salt or sugar means 6.02×10²³ molecules of them. The molecular weights of salt and sugar are 58.45 and 342.3, respectively. hence, sugar is heavier than salt. sugar molecules are bigger than the ions of dissolved salt and this is one of the reasons why sugar takes more time compared to salt for dissolving in any liquid.
Sugar, or sucrose, has the formula, C12H22O11. So one molecule has 45 atoms in it. The formula for salt is NaCl so one formula unit has 2 atoms. . Clearly, one molecule of sugar would be bigger than one formula unit of salt.
know more about microscopic and macroscopic representation :
https://brainly.com/question/13129524
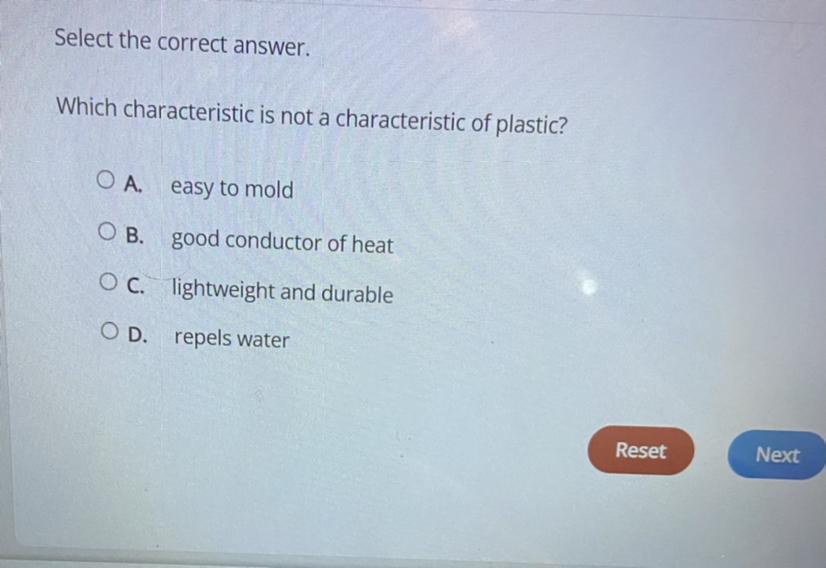
Which characteristic is not a characteristic of plastic?
A.) easy to mold
B.) good conductor of heat
C.) lightweight and durable
D.)Repels water
Image of question shown below
Answers
Answer:
I hope this helps
Explanation:

Carbon dioxide molecules (select all that apply)
Group of answer choices
Protect the Earth from all of the harmful Ultraviolet (UV) radiation
Absorb most of the shortwave radiation emitted from the Sun
Are one of the most abundant constituents of Earth's atmosphere
Can move in many ways, thus absorbing and emitting infrared radiation
Answers
Carbon dioxide molecules can absorb and emit infrared radiation, and they are one of the most abundant constituents of Earth's atmosphere.
Thus, the correct options are:d) Are one of the most abundant constituents of Earth's atmospheree) Can move in many ways, thus absorbing and emitting infrared radiation
Carbon dioxide is a trace gas present in the Earth's atmosphere. It's a vital component of Earth's carbon cycle, which helps to regulate Earth's temperature and support life as we know it. Carbon dioxide molecules are one of the most common gases in the atmosphere, accounting for around 0.04% of the Earth's atmosphere.
The greenhouse effect is caused by carbon dioxide, methane, and other greenhouse gases. When the Sun's energy reaches the Earth's surface, it is absorbed and then radiated back into space as infrared radiation. Greenhouse gases absorb this radiation and trap it in the atmosphere, which causes the Earth's temperature to rise and the climate to change.
Carbon dioxide molecules are capable of absorbing and emitting infrared radiation due to their molecular structure, which consists of one carbon atom and two oxygen atoms. This property of carbon dioxide is the main reason it's classified as a greenhouse gas.
To know more about Carbon dioxide molecules visit:
https://brainly.com/question/12770212
#SPJ11