Which taste sense causes sodium ion channels to open and generation an action potential?.
Answers
The taste sense that causes sodium ion channels to open and generate an action potential is known as the salty taste sense.
The salty taste is one of the five basic taste sensations experienced by humans, and it is caused by the presence of positively charged sodium ions in food or drinks. When these sodium ions come into contact with the taste buds on the tongue, they interact with sodium ion channels in the taste receptor cells, causing them to open.
The resulting influx of sodium ions into the cell generates an action potential, which then sends a signal to the brain via the gustatory nerves.
This signal is interpreted by the brain as a salty taste sensation.Therefore, it is the salty taste sense that causes sodium ion channels to open and generate an action potential.
To know more about acid visit :-
https://brainly.com/question/25148363
#SPJ11
Related Questions
HURRY!! Solve. C3H8(g) + 5O2(g) --> 3CO2(g) + 4H2O(g)
What is the Enthalpy of reaction?
What is the total Enthalpy of Reactants and Products? (Kj/Mol)
Is this a endothermic or exothermic reaction?
Answers
Answer: hope this helps!
Explanation:
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this series electrodes (two RC circuits in serie both are have the same values). This transfer function is defined as the measured pH (output) divided by the actual pH (input).
Obtain the analytical expression of the magnitude response of the system and plot the Bode plot of the system using MATLAB.
Obtain the analytical expression for h(t) and plot the impulse response of the electrode using MATLAB.
Obtain the analytical expression for the step response and plot it for the electrode using MATLAB.
All of this considering the RC series which contains the same values.
Please someone can help me with this questions. Thank you
Answers
In order to analytically find the transfer function, H(s), of the pH electrode, we can model it as a second-order passive low-pass filter consisting of two RC circuits in series.
The transfer function can be obtained by determining the ratio of the output voltage to the input voltage in the frequency domain.
Let's denote the Laplace transform variable as 's'.
The transfer function H(s) can be expressed as: H(s) = Vout(s) / Vin(s)
For a second-order passive low-pass filter, the transfer function can be written as:
H(s) = 1 / (s + s(R₁C₁ + R₂C₂) + R₁R₂C₁C₂)
Where R₁, R₂ are the resistances in the two RC circuits, and C₁ C₂ are the corresponding capacitances.
Now, let's assume the time constant
τ = R₁C₁ =R₂C₂ = 2 seconds (as given),
we can substitute this into the transfer function:
H(s) = 1 / (s² + 4s + 4)
Simplifying the transfer function further, we can factorize the denominator:
H(s) = 1 / ((s + 2)²)
So, the transfer function of the pH electrode, H(s), is:
H(s) = 1 / ((s + 2)²)
This transfer function represents the relationship between the measured pH (output) and the actual pH (input) of the electrode.
To know more about pH electrode here
brainly.com/question/28855246
#SPJ4
The complete question should be
The response of a pH electrode can be modeled as a first order or second order passive low pass filter (i.e. two RC circuits in series). A limitation of commercial pH electrodes is their slow response time, which is typically 2 seconds (i.e. = 2 s).
Analytically, find the transfer function, H(s), of this electrode. This transfer function is defined as the measured pH (output) divided by the actual pH (input).
Calculate the total number of protons, neutrons, and electrons for Bromine (Br).
Answers
Answer:
protons=35 neutrons=44.9 electrons=35
Explanation:

NaOH = CO2 -> Na2CO3 + H20
Solve the equation with steps
Answers
Answer:H2o
Explanation:
why do these molecules have similar bond angles but different names for their shapes?
Answers
Molecules with similar bond angles have similar geometries, or shapes, which are determined by the arrangement of atoms and electron pairs around a central atom. However, different molecules with similar bond angles may have different names for their shapes because the specific arrangement of atoms and electron pairs can result in unique geometric features. For example, molecules with four electron pairs around the central atom and a tetrahedral geometry may have different names depending on the type and arrangement of substituent atoms or groups attached to the central atom. Thus, while similar bond angles contribute to similar shapes, the specific arrangement of atoms and electron pairs can result in different names for the shapes of different molecules.
Molecules can have similar bond angles but different names for their shapes because the arrangement of atoms and the overall geometry of the molecule might differ even though the angles between the bonded atoms are the same. Bond angles are determined by the repulsion between electron pairs, while the shape of a molecule is defined by the spatial arrangement of atoms and their bonding. Let's consider two examples:
1. Bent shape - The water (H2O) molecule has a bent shape due to the presence of two lone pairs on the oxygen atom, causing the hydrogen-oxygen-hydrogen bond angle to be approximately 104.5 degrees.
2. Trigonal pyramidal shape - The ammonia (NH3) molecule has a trigonal pyramidal shape because of the presence of one lone pair on the nitrogen atom, which results in a nitrogen-hydrogen-hydrogen bond angle of approximately 107 degrees.
Even though the bond angles are quite similar (104.5 degrees and 107 degrees), the molecules are given different names for their shapes (bent and trigonal pyramidal) because the overall arrangement of atoms and their bonding patterns are not the same.
Learn more about bond angles : https://brainly.com/question/25425872
#SPJ11
254. A gas cylinder contains 0.722m³ of hydrogen gas at a pressure of 10.6 atm. If the
gas is used to fill a balloon at a pressure of 0.96 atm, what is the volume in m³ of the
filled balloon?
Answers
Answer:
2928281m² gas is used to fill a balloon at a pressure of 0.96 atm, what is the volume in m³ of the
filled balloon
Why is it necessary to have different types of equipment for the use in the laboratory?
Answers
Answer:
to make experiment simple
the average diameter of an adult human eyeball is 24mm what is the diameter in dm
Answers
Answer: 0.24 dm
Explanation:
Which of the following is the energy of motion? O Elastic energy O Gravitational energy O Kinetic energy O Potential energy
Answers
Answer:
kinetic energy
Explanation:
all moving objects have kinetic energy. when an object is an motion it changes its position by moving in a direction: up,down, forward, or backward
Answer: its C kinetic energy
Explanation: i did the test
The volume of a gas is increased and the temperature is maintained consent. The original volume was 1200 mm³ and it's pressure was 100psig. If the volume is increased to 2250 mm³ what is the new pressure in psi?
Answers
The formula for the ideal gas law is P*V = n*R*T, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
In this case, we are assuming that the number of moles and temperature are constant. Therefore, we can write:
P1*V1 = P2*V2, where P1 is the original pressure, V1 is the original volume, P2 is the new pressure, and V2 is the new volume.
We can solve for \(P2:P1*V1 = P2*V2P2\)
= P1*V1/V2Now we can substitute in the given values:
\(P1 = 100 psigV1 = 1200 mm³V2 = 2250 mm³P2 = 100 psig * 1200 mm³ / 2250 mm³P2 = 53.33 psig\)
Therefore, the new pressure is 53.33 psig.
To know more about formula visit:
https://brainly.com/question/20748250
#SPJ11
Which statement(s) is/are FALSE?
SELECT ALL THAT APPLY
The number of protons determines what type of
а
element an atom is.
b
Protons and neutrons repel each other and
position themselves far apart inside the atom.
Neutral atoms are made of specific numbers of
C protons and electrons but the number of neutrons
they possess can vary a little bit.
d All atoms are about the same size.
Answers
Answer:
D
Explanation:
not all atoms would be the same size. As for they vary scaleing from left to right in the periodic table.
Select the true statements about the citric acid cycle.
Answers
The citric acid cycle, also known as the Krebs cycle or TCA cycle, is a series of chemical reactions that occur in the mitochondria of eukaryotic cells and in the cytoplasm of prokaryotic cells.
The main function of the citric acid cycle is to oxidize acetyl-CoA, which is produced from the breakdown of carbohydrates, fats, and proteins, and generate energy in the form of ATP.
The first step of the citric acid cycle is the conversion of acetyl-CoA and oxaloacetate into citrate, which is catalyzed by the enzyme citrate synthase.
In each cycle of the citric acid cycle, one molecule of acetyl-CoA is completely oxidized to yield three molecules of NADH, one molecule of FADH2, one molecule of ATP or GTP, and two molecules of CO2.
The citric acid cycle is regulated by several enzymes, including citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase, which are allosterically regulated by ATP, ADP, and NADH.
The citric acid cycle is an important source of biosynthetic precursors, including amino acids, nucleotides, and heme.
The citric acid cycle is an anaerobic process that occurs in the absence of oxygen.
To learn more about citric acid visit:
brainly.com/question/28266073
#SPJ11
Separating sand and salt by filtration and evaporation, what are the observations in the experiment?
Answers
While Separating sand and salt by filtration and evaporation, the observation is during the filtration the sand gets accumulated in the filter paper while separating it from the salt solution, while in evaporation the salt gets accumulated.
When we tried to separate the individual components from the mixture of the salt and sand the first step is dissolving the mixture in water as the salt gets dissolved so it is easy for us to filter the sand from the solution, it is followed by the method of evaporation, in which the filtered salt solution is boiled until the water gets evaporated and we are left with the salt, that's how salt and sand are separated from a mixture.
To know more about evaporation refer to the link https://brainly.com/question/5019199?referrer=searchResults.
#SPJ9
Which fraction contains the largest molecules?
A) diesel
B)gasoline
C)kerosene?
Answers
PLEASE ANSWER!!! 30 POINTS
What mass of AI is needed to react with 72 g HCI?
2AI + 6HCI --> 2AICI3 + 3H
AI: 27 g/mol HCI: 36 g/mol
18 g HCI --> g AI
Answers
The mass of AI needed to react with 72 g of HCI is 54 g.
What is the mass of the AL needed?To determine the mass of AI needed to react with 72 g of HCI, we can use the stoichiometry of the balanced chemical equation you provided:
2AI + 6HCI --> 2AICI3 + 3H
From the equation, we can see that 2 moles of AI react with 6 moles of HCI to produce 2 moles of AICI3.
This means that the mole ratio between AI and HCI is 2:6 or 1:3.
Given the molar mass of HCI is 36 g/mol, we can calculate the number of moles of HCI in 72 g of HCI by dividing 72 g by the molar mass of HCI:
Number of moles of HCI = mass of HCI / molar mass of HCI
Number of moles of HCI = 72 g / 36 g/mol
Number of moles of HCI = 2 moles
Since the mole ratio between AI and HCI is 1:3, the number of moles of AI needed to react with 2 moles of HCI is also 2 moles.
Now, we can use the molar mass of AI, which is 27 g/mol, to calculate the mass of AI needed to react with 2 moles of HCI:
Mass of AI = number of moles of AI × molar mass of AI
Mass of AI = 2 moles × 27 g/mol
Mass of AI = 54 g
Learn more about reactant mass here: https://brainly.com/question/26682140
#SPJ1
How does a cirrus cloud look like
1) cotton
2) thin feather
Answers
Answer:
2) thin feather
Explanation:
Cirrus clouds are delicate, feathery clouds that are made mostly of ice crystals. Their wispy shape comes from wind currents which twist and spread the ice crystals into strands.
hope it helps :)
please mark brainliest!!!
Put the following in order of most to least. (Hint: convert all to their base unit and then compare.)
20 kilograms
8,776,674,266 micrograms
500 decagrams
1 gigagram
2 teragrams
Answers
The arrangement or order of the mass measurement from the most to the least is 2 teragrams, 1 gigagram, 20 kilograms, 8,776,674,266 micrograms, and the least is 500 decagrams.
What is unit conversion?
Unit conservation is a process in which unit of an object is converted from one form to another.
The unit of mass of the substance is converted as follows;
20 kilograms = 20 kg x 1000 g/kg = 20,000 g
8,776,674,266 micrograms = 8,776,674,266 μg = 8,776,674,266 x 10⁻⁶ g = 8,776.674266 g
500 decagrams = 500 Dg = 500 x 10 = 5,000 g
1 gigagram = 1Gg = 1 x 10⁹ g
2 teragrams = 2 x 10¹² g
The order of the mass of the substance from the most to the least.
2 teragrams1 gigagram20 kilograms8,776,674,266 micrograms500 decagramsThus, the arrangement or order of the mass measurement from the most to the least is 2 teragrams, 1 gigagram, 20 kilograms, 8,776,674,266 micrograms, and the least is 500 decagrams.
Learn more about unit of mass here: https://brainly.com/question/20024683
#SPJ1
what are the answers?

Answers
·· ··
The balanced equation was found to be Na· + ·Cl : ---------> Na + (: cl : )-
.. ..
Define ionic bond dot diagram ?
Group 8 elements, generally known as noble gases, are noted for their inertness. They hardly never react with other elements, such as water, oxygen, or metals. This is due to the fact that they all have a complete outer shell of electrons. This is the most stable electron configuration possible for an atom.
Other elements, on the other hand, do not have this stable electron configuration. Instead, in order to obtain that ideal arrangement, they must acquire, lose, or share electrons. Ionic bonding is one method for doing this.
the answer for the ionic bond dot diagram for the Nacl was found to be.
·· ··
Na· + ·Cl : ---------> Na + (: cl : )-
.. ..
To learn more about ionic bond follow the given link: https://brainly.com/question/13526463
#SPJ1
The density of liquid mercury is 13.6 g/mL. What is the mass of a 175 mL sample of mercury?
Answers
Answer:
The answer is
2380 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density of mercury = 13.6 g/mL
volume = 175 mL
The mass of the metal is
mass = 13.6 × 175
We have the final answer as
2380 gHope this helps you
Please answer 50 points!!!! IT'S URGENT
N₂(g) + 3H₂(g) → 2NH3(g)
224 L of nitrogen reacts with excess hydrogen at 2773 K and 95.0 atm. How many moles of nitrogen react?
Use Pv=nRT
Answers
The number of moles of nitrogen in reaction with hydrogen at the given condition is 93.5 moles of N₂.
What is the number of the nitrogen that reacts?The number of moles of nitrogen in reaction with hydrogen at the given condition is calculated as follows;
N₂(g) + 3H₂(g) → 2NH₃(g)
Number of moles of H₂ in the balanced equation = (3/1) x Number of moles of N₂
Apply ideal gas law as follows;
PV = nRT
where;
P is the pressureV is the volumen is the number of molesR is the gas constantT is the temperature in kelvinsn = PV/RT
n = (95.0 atm) x (224 L) / [(0.0821 L.atm/mol.K) x (2773 K)]
n = 93.5 moles of N₂
Learn more about number of moles here: https://brainly.com/question/14357742
#SPJ1
Which of the following could potentially have a mutation? (Check all that may apply.)
Fungi
Animals
Bacteria
Viruses
Plants
Answers
Answer:
all of them
Explanation:
What is the reaction to . Na2SO3 + 2 HC2H3O2 → H2SO3 + 2 NaC2H3O2
Answers
How many moles are 1.20x10^25 atoms of phosphorus. Look at the picture

Answers
Answer:
19.9 moles
Explanation:
Each mole has 6.02 x 10^23 many atoms. This number is commonly known as "Avogadro's Number."
In this problem, we have 1.2 x 10^25 atoms of phosphorus.
Thus, (1.2 x 10^25)/(6.02 x 10^23) is the number of moles in 1.20 x 10^25 many atoms of phosphorus.
(1.2(10^25))/(6.02(10^23))
19.9 moles.
The moles are 19.9 moles
The calculation is as follows:
Each mole has \(6.02 \times 10^{23}\) many atoms.
This number is commonly known as "Avogadro's Number."
In this given question, we have \(1.2 \times 10^{25}\) atoms of phosphorus.
So \((1.2 \times 10^{25})\div (6.02 \times 10^{23})\)is the number of moles in\(1.20 \times 10^{25}\) many atoms of phosphorus.
Now
\((1.2(10^{25}))\div (6.02(10^{23}))\)
19.9 moles.
Learn more; https://brainly.com/question/17429689?referrer=searchResults
An unknown liquid occupies a volume of 5 ml and has a mass of 40 grams. Find its density.
Answers
Answer:8g mL^-1
Multiple 1mL * 40g/5mL
If an atom has 3 protons, what is its atomic number?
Answers
Answer:
Atomic Number = 3.
Explanation:
The number of protons determines the atomic number. Basically the number of protons is the exact same as the atomic number.
Protons 3 = Atomic number 3
What element does Chlorine go in?
- metals
-not metals
-Metalloids
Answers
Answer:
non of them it is
halogen elements
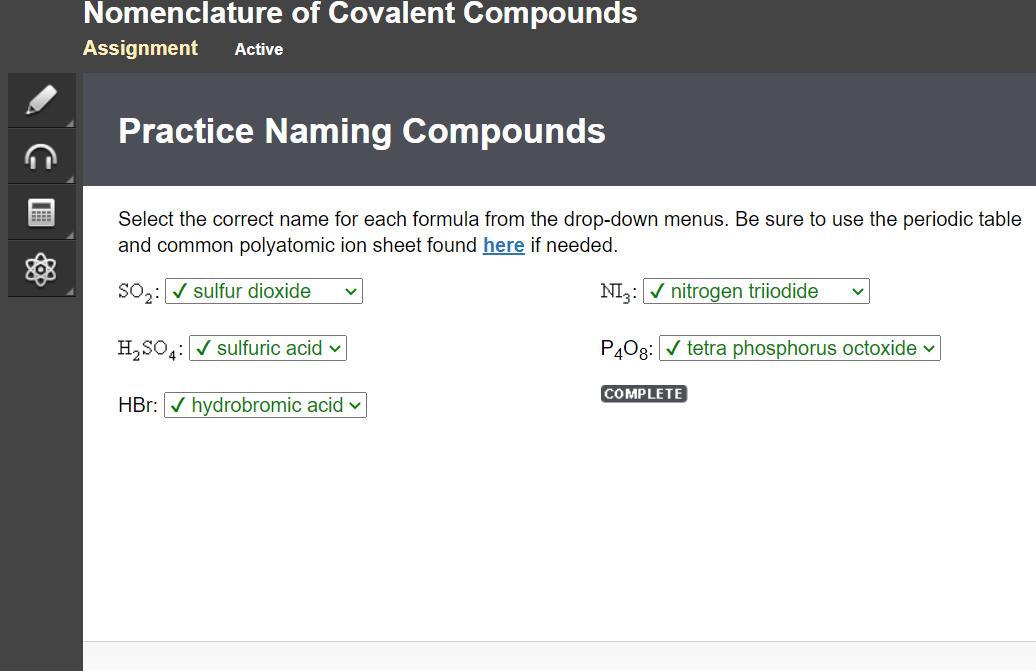
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

Which of the following statements is true concerning acids and bases?
1-acids mixed with bases neutralize each other
2-acids mixed with bases make stronger acids
3-acids mixed with bases make stronger bases
4-acids and bases don't react with each other
Answers
The true statement about acids and bases is; acids mixed with bases neutralize each other. Option 1 is correct.
When an acid reacts with a base, they undergo a chemical reaction called neutralization, resulting in the formation of water and a salt. The hydrogen ions (H⁺) from the acid react with the hydroxide ions (OH⁻) from the base to form water (H₂O), while the remaining ions from the acid and the base combine to form a salt.
The neutralization reaction between acids and bases results in the formation of a neutral solution, with a pH close to 7. This is because the acidic and basic properties of the original substances are cancelled out or neutralized by each other.
Hence, 1. is the correct option.
To know more about neutralization here
https://brainly.com/question/15347368
#SPJ1
a liter of water was found to contain 10 mg of benzene (c6h6). what is the concentration of benzene in molarity (m)?
Answers
The concentration of benzene in molarity (M) is calculated by dividing the amount of benzene (10 mg) by its molar mass (78.11 g/mol). This gives a concentration of 0.128 M.
To calculate the concentration of benzene in molarity (M), you need to divide the amount of benzene (10 mg) by its molar mass (78.11 g/mol). This gives the concentration of benzene as 0.128 M. The molar mass of a substance is the mass of one mole of the substance, which is equal to Avogadro's number of molecules of the substance.
In this case, one mole of benzene is equal to 78.11 g, and 10 mg of benzene is equal to 0.128 moles. Therefore, dividing 10 mg by 78.11 g/mol gives 0.128 moles, which is equal to 0.128 M.
Learn more about The concentration of benzene:
https://brainly.com/question/22016139
#SPJ4
An electric kettle draws a current of 6.50 A while it is plugged into a 120-V
electrical outlet. What power does the kettle use?
Answers
Answer:
780 watts
Explanation:
formula to find power when given amps and voltage
P = A x V
=6.50a x 120V
= 780 W (watts)