Which sentence contains a double negative?
He could hardly catch his breath.
I know nothing about computers.
Sharon was unaware of her errors.
She never danced with nobody. pls helppppp
Answers
She never danced with nobody.
Explanation:
The double negative is intended to convey to the reader a negative sentiment while emphasizing and reinforcing that notion. The double negative may be seen in sentences that include two words that have a negative connotation yet are in perfect harmony with the other words in the phrase as well as with the sentences' coenrency and cohesiveness.
There are two negative terms in the sentence "She never danced with nobody." These are the words "never" and "nobody," which are perfectly in sync with the rest of the sentence's components. So, here's an excellent example of a double negative statement.
#SPJ1
Related Questions
HELP!!! URGENT!!! I WILL GIVE BRAINLIEST TO THE FIRST RESONABLE ANSWER!!! PLEASE EXPLAIN IN A SIMPLE FORMAT!
Why does a larger object like earth have a greater pull of gravity?
A - It doesn't have a greater pull
B - The larger object has more friction
C - The larger object has more speed
D - The larger object has more mass
Answers
Answer:
D-The larger object has more mass
Explanation:
The more mass something has, the more gravitational pull. Think of if you and a sumo wrestler sat on a giant trampoline that's covered in marbles (except the trampoline is the fabric of space time but that's not important). Who's going to sink down more and pull more marbles towards them? The sumor westler because they have more mass and therefore more gravitational pull.
Consider a group of 5 students, each of whom makes a potassium chloride (74.55 mol/g) solution in the lab. Individually, each student weighs out an amount of sodium chloride and a volume of water, according to the following table. Use this information to calculate the solution concentration (in moles/liter) each student makes, the average concentration, and the standard deviation for the concentration.
fill out the chart pls

Answers
Normal Concentration = 18.09 moles/L Standard Deviation = 1.89 moles/L
What is Concentration?The capacity for sustained attention on a single thing, activity, or work without being sidetracked is known as concentration. It is the key to success in most endeavors, as it requires a person to ignore any extraneous thoughts, feelings, or stimuli and instead focus on the task at hand. Concentration is a mental skill that can be developed and improved with practice, such as with mindfulness, meditation, and various cognitive exercises. Concentration can also be adversely affected by poor physical health, lack of sleep, and stress, so it is important to maintain a healthy lifestyle if one wants to stay focused.
Student | Weight (g) | Volume (L) | Concentration (moles/L)
1 | 5.0 | 0.25 | 19.82
2 | 8.0 | 0.50 | 15.89
3 | 4.0 | 0.20 | 20.23
4 | 7.0 | 0.30 | 18.52
5 | 6.0 | 0.40 | 16.96
To learn more about concentration
https://brainly.com/question/26255204
#SPJ1
Urgent! I'm Begging You!!! 100 points! I Just need something to write!
Manipulate: Recall that molarity is equal to the number of moles of a substance dissolved in one liter of solution: molarity = moles ÷ volume.
A. How many mL of NaOH did you add?
B. Using: molarity = moles/volume, how many mol NaOH was added to the flask?
C. Using the mole ratio, determine the number of mol of H2SO4?
D Now calculate [H2SO4] using molarity = moles/volume
Answers
We need to use the formula for molarity, which relates the number of moles of a substance to the volume of solution.
From there, we can use mole ratios and additional calculations to determine the number of moles and concentration of another substance in the solution.
A. To determine how many mL of NaOH were added, we need to know the volume of the solution in which it was dissolved. This information is not given in the question, so we cannot provide a specific answer.
B. Using the formula molarity = moles/volume, we can calculate the number of moles of NaOH that were added to the flask. We need to know the molarity of the NaOH solution and the volume of NaOH that was added. Again, this information is not provided in the question, so we cannot give a specific answer.
C. To determine the number of moles of H2SO4 in the solution, we need to use the mole ratio between NaOH and H2SO4. This is because when NaOH and H2SO4 react, they do so in a specific proportion according to their balanced chemical equation. If we know the number of moles of NaOH that were added (from part B), we can use the mole ratio to calculate the number of moles of H2SO4 present.
D. Finally, we can calculate the concentration of H2SO4 in the solution using the formula molarity = moles/volume. If we know the number of moles of H2SO4 (from part C) and the volume of the solution, we can use this formula to determine the molarity of H2SO4 in the solution.
Overall, the answer to this question requires multiple pieces of information that are not provided. Therefore, we cannot give a specific answer and can only provide a general explanation of the steps involved in solving the problem.
To begin, we need to understand the formula for molarity, which is defined as the number of moles of a substance dissolved in one liter of solution. This formula is expressed as:
molarity = moles ÷ volume
Using this formula, we can determine the molarity of a solution if we know the number of moles of a substance present and the volume of the solution.
Now, let's look at the specific questions that were asked in this problem:
A. How many mL of NaOH did you add?
Unfortunately, we cannot answer this question because the volume of the NaOH solution that was added is not provided in the question.
B. Using: molarity = moles/volume, how many mol NaOH was added to the flask?
Similarly, we cannot answer this question because the molarity of the NaOH solution and the volume of NaOH that was added are not given in the question.
C. Using the mole ratio, determine the number of mol of H2SO4?
To answer this question, we need to use the balanced chemical equation for the reaction between NaOH and H2SO4:
NaOH + H2SO4 → Na2SO4 + 2H2O
From this equation, we can see that one mole of NaOH reacts with one mole of H2SO4. Therefore, if we know the number of moles of NaOH that were added (from part B), we can use this mole ratio to determine the number of moles of H2SO4 that are present.
For example, let's say that we added 0.1 moles of NaOH to the flask. Since the mole ratio between NaOH and H2SO4 is 1:1, we know that there are also 0.1 moles of H2SO4 present in the solution.
D. Now calculate [H2SO4] using molarity = moles/volume
Finally, we can use the formula for molarity to calculate the concentration of H2SO4 in the solution. If we know the number of moles of H2SO4 (from part C) and the volume of the solution, we can use the following formula:
molarity = moles ÷ volume
For example, let's say that the volume of the solution is 500 mL. To calculate the molarity of H2SO4, we can divide the number of moles of H2SO4 (0.1) by the volume of the solution (0.5 L or 500 mL):
molarity = 0.1 moles ÷ 0.5 L = 0.2 M
Therefore, the concentration of H2SO4 in the solution is 0.2 M.
In summary, the answer to this question requires multiple pieces of information that are not provided.
To know more about molarity visit:
brainly.com/question/8732513
#SPJ11
Why are certain amino acids defined as essential for human beings?
Select one alternative:
Because human beings do not have biochemical pathways to synthesize these amino acids from simpler precursors
Because human beings do not have biochemical pathways to break down these amino acids from more complex precursors
Because human beings do not have enough protein to synthesize these amino acids
All statements are true
Answers
The correct alternative is: Because human beings do not have biochemical pathways to synthesize these amino acids from simpler precursors.
Certain amino acids are defined as essential for human beings because our bodies do not have the necessary biochemical pathways to synthesize these amino acids from simpler precursors. These essential amino acids need to be obtained from the diet to ensure proper growth, development, and overall health.
Amino acids are the building blocks of proteins, and they play crucial roles in various biological processes. There are 20 different amino acids that can be combined to form proteins. Among these, nine amino acids are classified as essential for humans: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Our bodies have the ability to synthesize non-essential amino acids, which can be produced from other molecules or through metabolic pathways. However, essential amino acids cannot be synthesized by our bodies in sufficient quantities or at all, which is why they must be obtained through dietary sources.
These essential amino acids play important roles in protein synthesis, enzyme function, hormone production, and various physiological processes. Inadequate intake of essential amino acids can lead to protein deficiency and impaired growth, muscle wasting, weakened immune function, and other health problems.
The conclusion is that Certain amino acids are classified as essential for human beings because our bodies lack the biochemical pathways required to synthesize them from simpler precursors. Therefore, it is necessary to obtain these essential amino acids through the diet to maintain optimal health and physiological functioning.
Learn more about amino acids here https://brainly.com/question/31442968
#SPJ11
What's the name of this molecule?

Answers
Answer:
option d
Explanation:
Idk what the answer is, can someone help?

Answers
2
3
4
20
Is the answer for the question
does sodium chloride retain any characteristic of sodium or chlorine?
Answers
Answer:
When this occurs, sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative. Opposite charges attract, right? So then, sodium ions will attract chloride ions and form an ionic bond.
Explanation:
Please Help
Predict the nature of the indicated covalent
bond. Polar or Non polar
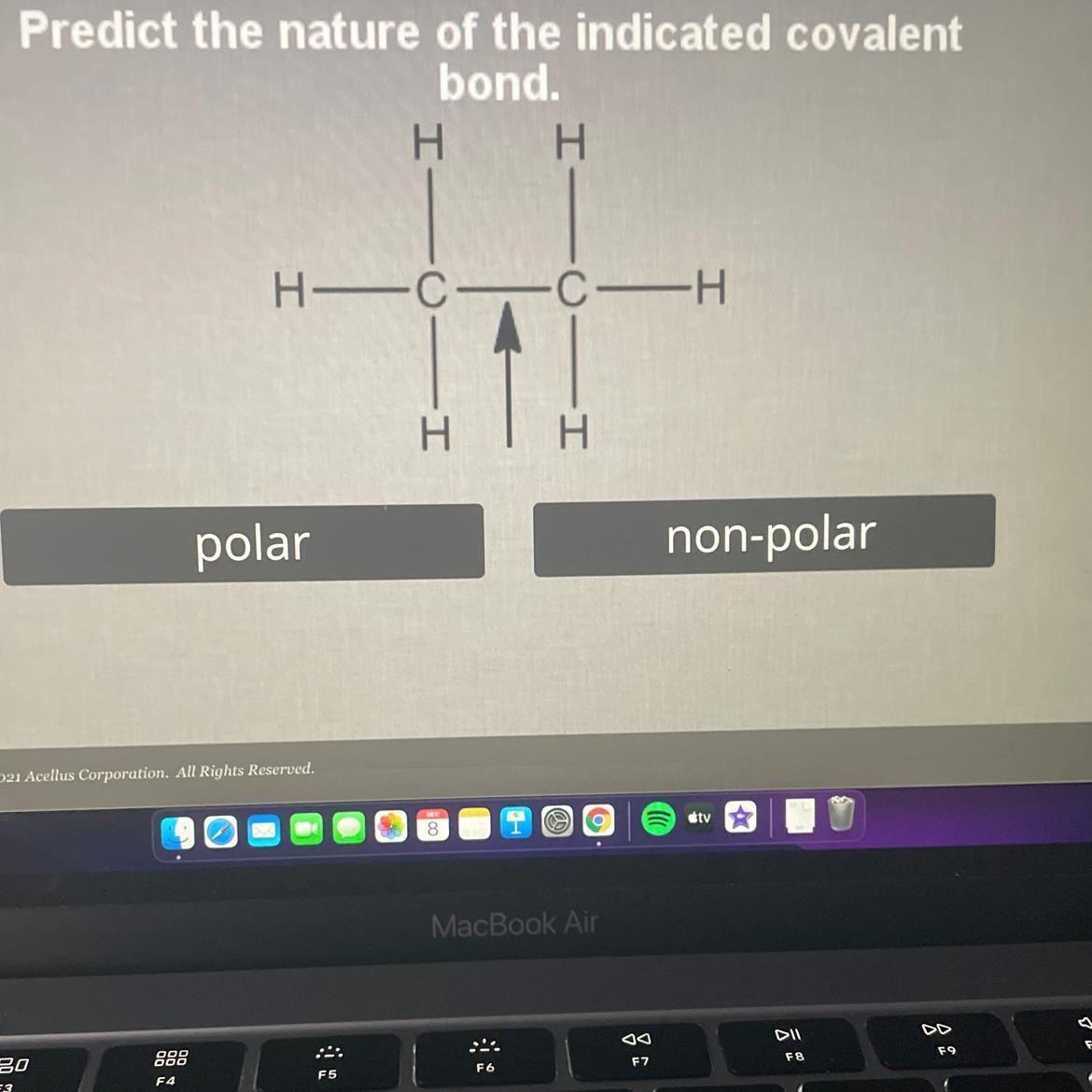
Answers
Answer:
Nonpolar
Explanation:
which best represents the protonation state of the amino acid cysteine at ph=7
Answers
The protonation state of cysteine at pH 7 is a zwitterion.
Cysteine is an amino acid that contains a thiol group (-SH) in its side chain. At physiological pH (around 7), cysteine exists in a zwitterionic form. A zwitterion is a molecule that carries both positive and negative charges, but overall has a net neutral charge. In the case of cysteine, the thiol group (-SH) can lose a proton (H⁺) and become negatively charged (-S⁻), while the amino group (NH₂) can accept a proton (H+) and become positively charged (+NH₃⁺). This results in a molecule with both positive and negative charges, making it a zwitterion.
The pH of a solution determines the concentration of protons (H⁺ ions) present. At pH 7, the concentration of protons is near neutral, and the thiol group tends to lose a proton, becoming negatively charged (-S⁻). Similarly, the amino group accepts a proton, becoming positively charged (+NH₃⁺). These charges balance each other out, leading to the formation of a zwitterion.
Cysteine's zwitterionic form is crucial for its role in protein structures and functions. The thiol group of cysteine can form disulfide bonds with other cysteine residues, contributing to the stabilization of protein structures. Additionally, the charged nature of the zwitterion allows cysteine to participate in various biochemical reactions, such as redox reactions and metal ion coordination.
Learn more about cysteine
https://brainly.com/question/32350816
#SPJ11
What are the alkali metals?
Answers
Answer:
Are the six chemical elements
Explanation:
Answer:
they are the first group in the periodic table
Explanation:
its on the far left of the table- this is the apex answer
How much 0.05 M HCl solution can be made by diluting 2L of 10 M HCl?
Answers
Answer:
400 L
Explanation:
As this problem deals with a dilution process, we can use the following formula:
C₁V₁=C₂V₂Where subscript 1 stands for the concentrated concentration and volume, while 2 stands for the diluted values. Meaning that in this case:
C₁ = 10 MV₁ = 2 LC₂ = 0.05 MV₂ = ?We input the data:
10 M * 2 L = 0.05 M * V₂And solve for V₂:
V₂ = 400 LMass (g) of 8.35 mol of copper(I) carbonate
Answers
Mass of Cu₂CO₃ = moles × molar mass
= 8.35 mol × 187.1 g/mol
= 1562.29 g
∴ the mass of 8.35 mol of copper(I) carbonate is 1562.29 g.
How many different signals would you expect to see in the 1H NMR of the given compounds? (i.e. different chemical shifts)? 10 Compound A- Compound B- 6 Compound C- 4 6 Compound D- 9 Compound E- Compound F-
Answers
Nuclear magnetic resonance spectroscopy (NMR) is used to study the electronic environment of atoms and the bonding nature of a compound.
The number of different signals observed in the 1H NMR spectra of the given compounds are as follows: Compound A: 10 signals, Compound B: 6 signals ,Compound C: 4 signals Compound D: 6 signals ,Compound E: 9 signals, Compound F: 3 signals
In proton nuclear magnetic resonance spectroscopy (1H NMR), the magnetic field strength and electronic environment of the protons influence the chemical shifts. The shielding effect or deshielding effect of the nearby atoms, bond length, and bond angle, among other factors, all influence the chemical shifts. There are five different types of proton environments, which correspond to five different chemical shifts, in this case: Type 1: Hydrogen atoms that are covalently bonded to sp3 hybridized carbons. Type 2: Hydrogen atoms that are covalently bonded to sp2 hybridized carbons. Type 3: Hydrogen atoms that are covalently bonded to sp hybridized carbons. Type 4: Hydrogen atoms that are covalently bonded to aromatic carbons. Type 5: Hydrogen atoms that are directly bonded to electronegative elements such as nitrogen, oxygen, or halogens.
Here, to determine the number of signals expected in the 1H NMR of the compounds. Compound A: 10 different proton environments => 10 signals. Compound B: 6 different proton environments => 6 signals. Compound C: 4 different proton environments => 4 signals. Compound D: 6 different proton environments => 6 signals. Compound E: 9 different proton environments => 9 signals. Compound F: 3 different proton environments => 3 signals.
Let's learn more about NMR:
https://brainly.com/question/17564948
#SPJ11
Write balanced chemical equations as indicated for any observed reactions. Write NR if no reaction was observed. Saved Normal BIITU X, X 15 fx lo x A. Overall equation for Ba(NO3)2 and Na2CO3. Ba(NO3)2 + Na2CO3- BaCO3 + NaNO3 B. Ionic equation for Ba(NO3)2 and Na2CO3: C. Net-ionic equation for Ba(NO3)2 and Na2CO3: Ba(NO3)2 + Na2CO3- BaCO3 + NaNO3 D. Overall equation for Ba(NO3)2 and Na3P04: Ba(NO3)2 + Na3PO4-Ba3(PO4)2 + NaNO3 E. Ionic equation for Ba(NO3)2 and NaCl: NR F. Net-ionic equation for Ba(NO3)2 and NaOH!
Answers
All the chemical equations of this question has been balanced for the given observed reactions.
Equal numbers and types of each atom appear on both sides of balanced chemical equations. A balanced equation must have coefficients that are the simplest whole number ratio. Chemical reactions always conserve mass.
A. Ba(NO₃)₂+ Na₂CO₃ → BaCO₃ + 2NaNO₃
B. Ba₂⁺ + 2NO₃⁻ + 2Na⁺ +CO₃²⁻→ BaCO₃(s) +2Na⁺ +2NO³⁻
C. Net ionic equation-
Ba²⁺(aq) + CO₃²⁻(aq)→ BaCO₃(s)
D. overall reaction-
3Ba(NO₃)₂(aq) + 2Na₃PO₄→ Ba₃(PO₄)₂(s) + 6NaNO₃(aq)
E. Ionic equation-
Ba(NO₃)₂(aq) + 2NaCl(aq)→ BaCl₂(aq) + 2NaNO₃(aq)
∴ No reaction
F- Ba(NO₃)₂(aq) + 2NaOH(aq) → Ba(OH)₂(aq) +2NaNO₃(aq)
∴No net ionic equation.
Thus, all equations are balanced above.
To learn more about Balanced chemical equation refer-https://brainly.com/question/26227625
#SPJ4
a gas under constant pressure will ____________ when ____________.
Answers
A gas under constant pressure will expand when heated.
What is temperature?
The temperature is the degree of hotness or coldness of the object.
According to the ideal gas law.
pV= nRT
where, p = pressure. V = volume, n =moles of elements, R =universal gas constant and T =temperature.
When pressure is kept constant, volume is directly proportional to the temperature.
When temperature is increased or an object is heated, the volume also increases i.e expands.
Thus, a gas under constant pressure will expand when heated.
Learn more about temperature.
https://brainly.com/question/7510619
#SPJ4
chemical porperties of synthetic fiber
Answers
Explanation: Some of the most important properties of synthetic materials are as follows: 1. Tensile strength 2. The Action of water 3. The Action of heat and flame 4. Thermal conductivity 5. Electrical conductivity. The usefulness or otherwise of a synthetic material depends upon the following properties. 1. Tensile strength:
PLEASE MARK ME AS THE BRAINLIEST
1. Consider NH3.If it dissolves in water(i) NH3 + H20 + NHẤ4+ H2O(ii)NH3 + H2O → NH+3 + OH-(iii) NH3 + H2O + NH+4+ OH-(iv) NH3 + H2O → NH+4+ OH-Which represents the dissolution of NH3 in water(a) i(b) ii (c) iii (d) iv (e) iii and iv2. HOA2+H20 . → H3O+ + OA-CIn this reaction:(i) OA c is the conjugate base of H2O(ii)OA-c is the conjugate base of HOAc (iii) H3O+ is theсconjugate base of HOA.(iv) H3O+ is the conjugate acid of H2O(a) i(b) ii (c) iii (d) iv (e) none3. Arrange the following according to increasing acid strength(i) Ka= 2.5 + 10-15(ii) Ka= 9.0 + 10-9(iii) pKa= 7.5(iv) % dissociation =100(a) iv, iii, ii, i2(b) ii, I, iii, iv(c) i, iii, iv, ii(d) i, ii, iii, iv(e) iii, iv, ii, i2
Answers
1. Ammonia is a colorless gas with a chemical formula of NH3, when it comes in contact with water, it will be transformed into Ammonium ion and it will produce one hydroxide ion, and this is why Ammonia will present a more basic (pH) behavior, the reaction that represents this behavior is:
NH3 + H2O -> NH4+ + OH-
Number 4 is the only one that represents it well
Number 3 has the same reaction but since there is a plus sign instead of an arrow, I consider it wrong.
the pH of a solution is 2.0. what is the [OH^-] concentration?
Answers
draw the major organic product of the reaction, which has the molecular formula c7h9n.
Answers
Unfortunately, the given molecular formula (C7H9N) is not sufficient to uniquely determine the starting material or the reaction conditions.
There are many different organic compounds with the same molecular formula, and the reactivity of these compounds can vary widely depending on the reaction conditions. To predict the major organic product of a reaction, we would need to know the starting material and the specific reaction conditions (e.g. reagents, solvents, temperature, etc.). Once we have this information, we can apply our knowledge of organic chemistry to predict the likely reaction pathway and the major product(s) that would be formed. If you can provide more information about the starting material and the reaction conditions, I would be happy to try and help you predict the major organic product.
To learn more molecular formula click the link below
brainly.com/question/28647690
#SPJ4
What is the definition of a scientific theory
A) A method of learning that emphasizes reason as the way to understand the world.
B) A brief statement that summarizes past observations and predicts future ones.
C) A model that explains the underlying reasons for observations and laws.
D) The equivalent of a scientific opinion which others may disagree with.
E) None of these.
Answers
Answer:
The correct answer to the following question will be Option C.
Explanation:
The theory seems to be the context within which findings are explained as well as possible predictions are made. This theoretical perspective discusses the factors behind the results as well as the laws.Theories have been used in scientific knowledge to give wide interpretations to accommodate observed facts in case the root reason is still yet to be found as well as characterized.So that Option C will be the right answer.
Which of the following is a physical change?
a. burning gasoline
b. cooking an egg
c. decomposing meat
d. evaporating water
Answers
enough of a monoprotic acid is dissolved in water to produce a 1.48 m solution. the ph of the resulting solution is 2.80 . calculate the ka for the acid.
Answers
The Ka value for the monoprotic acid on dissociation is found to be 16.44.
The dissociation of monoprotic acid HA is as,
HA = H⁺ + A⁻
So, the Ka value is defined as,
Ka = [H⁺][A⁻]/[HA]
The pH of a compound is given as,
log([H⁺]) = pH
The given pH is 2.8, so,
log([H⁺]) = 2.8
[H⁺] = 16.44 M.
Now, as we can see from the equation,
[H⁺] = [A⁻] = [HA]
So, putting the values for Ka,
Ka = [H⁺][A⁻]/[HA]
Ka = 16.44×16.44/16.44
Ka= 16.44
So, the value of Ka for this acid is 16.44.
To know more about pH value of the solution, visit,
https://brainly.com/question/172153
#SPJ4
is change color when mixed with water a physical or chemical property?
Give reasons for your answer.
Answers
Explanation:
Color. The changing of color of a substance is not necessarily an indicator of a chemical change. For example, changing the color of a metal does not change its physical properties. However, in a chemical reaction, a color change is usually an indicator that a reaction is occurring.
which statement describes a mixture
Answers
Answer:
Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup.
The data below were collected for the following reaction: 2NO2(g)+F2(g)?2NO2F(g)
[NO2](mol L?1) [F2](mol L?1) Initial Rate (mol L?1 s?1)
0.100 0.100 0.026
0.200 0.100 0.051
0.400 0.400 0.411
A) Write an expression for the reaction rate law.
Write an expression for the reaction rate law.
Rate=k[NO2][F2]
Rate=k[NO2]
Rate=k[F2]
Rate=k[NO2]2[F2]
B) calculate the value for the rate constant, k
C) What is the overall order of the reaction?
Answers
A) The expression for the reaction rate law can be written as: Rate=k[NO2][F2]
B) To calculate the value for the rate constant, we need to choose any set of concentrations and corresponding rates from the data provided and substitute them in the rate law expression. Let's use the first set of concentrations and rates:
Rate = k[NO2][F2]
0.026 mol L^-1 s^-1 = k(0.1 mol L^-1)(0.1 mol L^-1)
k = 0.026 mol L^-1 s^-1 / (0.1 mol L^-1)(0.1 mol L^-1)
k = 2.6 L^2 mol^-2 s^-1
C) The overall order of the reaction is the sum of the orders with respect to each reactant. In this case, the reaction rate law is first order with respect to both [NO2] and [F2]. Therefore, the overall order of the reaction is 1 + 1 = 2.
In summary, the expression for the reaction rate law is Rate=k[NO2][F2], the rate constant (k) was calculated to be 2.6 L^2 mol^-2 s^-1 using the initial rate data, and the overall order of the reaction is 2 since it is the sum of the orders with respect to each reactant.
To learn more about reaction click here: brainly.com/question/30464598
#SPJ11
Can two objects be made of the Same substance if they have the same volume
Answers
Answer: no
Explanation: theres a chance but generally no because there can be different substances in different amounts which can have similar volumes
Which energy changes are associated with a liquid boiling? Energy is released, and potential energy decreases. Energy is absorbed, and potential energy increases. Energy is released, and kinetic energy decreases. Energy is absorbed, and kinetic energy increases.
Answers
Answer: Energy is absorbed, and kinetic energy increases
Explanation:
Pls mark brainliest :)
115 mL of a 2.35 M potassium
fluoride (KF) solution is diluted with 1.28 L of water. What is the new concentration in molarity?
? M KF
Answers
The new concentration : = 0.194 M
Further explanationGiven
115 mL(0.115 L) of a 2.35 M Potassium fluoride (KF)
1.28 L of water
Required
The new concentration
Solution
Dilution is the process of adding solvent to get a more dilute solution.
The moles(n) before and after dilution are the same.
Can be formulated :
M₁V₁=M₂V₂
M₁ = Molarity of the solution before dilution
V₁ = volume of the solution before dilution
M₂ = Molarity of the solution after dilution
V₂ = volume of the solution after dilution
V₂=0.115 + 1.28 = 1.395 L
Input the value :
M₂ = (M₁V₁)/V₂
M₂ = (2.35 x 0.115)/1.395
M₂ = 0.194
Answer: 0.194
Explanation:
When sulfuric acid is added to solid sodium chloride and the mixture is heated, hydrogen chloride gas is generated, leaving a residue of sodium sulfate
Answers
The balanced chemical equation for the reaction between sulfuric acid and sodium chloride to produce hydrogen chloride gas and sodium sulfate is:
2H₂SO₄ + 2NaCl -> 2HCl + Na₂SO₄
What is the equation about?The equation given above is:
Sulfuric acid + sodium chloride -> hydrogen chloride + sodium sulfate
Therefore, In this equation, sulfuric acid (H₂SO₄) is the acid and sodium chloride (NaCl) is the base. The acid donates protons (H+ ions) to the base, resulting in the production of hydrogen chloride gas (HCl) and a salt (Na₂SO₄).
The overall reaction is an example of an acid-base reaction, in which the acid and base react to produce a salt and a neutralization product (in this case, HCl).
Learn more about balanced chemical equation from
https://brainly.com/question/26694427
#SPJ1
See full question below
When sulfuric acid is added to solid sodium chloride and the mixture is heated, hydrogen chloride gas is generated, leaving a solid residue of sodium sulfate. Give the balanced chemical equation for this reaction.

Butyric acid is a weak acid. Which reaction equation properly represents the dissociation of butyric acid in water?
Answers
The reaction equation properly represents the dissociation of butyric acid in water is CH3CH2COOH+H2O⇔ CH3CH2CH2COO- +H3O.
Dissociation would be a basic method in chemistry and biology when molecules dissociate or split into some other substances like atoms, ions, or radicals, typically in a reversible manner.
When an acid is dissolved in an aqueous solution, it separates into hydrogen ions (\(H^{+}\)) as well as anions. Strong acids produce a lot of H+ due to the molecules' dissociation.
Therefore, The reaction equation properly represents the dissociation of butyric acid in water is\(CH_{3} CH_{2} COOH\)+\(H_{2} O\)⇔ \(CH_{3} CH_{2} CH_{2} COO^{-}\)+\(H_{3} O.\)
To know more about butyric acid
https://brainly.com/question/13505231
#SPJ4