Which of the following is a limitation of Bohr's model?
Bohr's model failed to explain the behavior of any atom
Bohr's model could not explain how electrons became excited
Bohr's model was able to explain the chemical behavior of only non-metals
Bohr's model was not able to explain the chemical behavior of any atom besides hydrogen
Answers
Answer:
The answer is:
The first option
Explanation:
Related Questions
a myelin sheath is a fatty layer that protects the axon, so it is most like the
Answers
A myelin sheath is most like the insulation around an electrical wire.
The myelin sheath is a protective layer that surrounds and insulates the axon of a nerve cell. It is composed of fatty substances called lipids, specifically phospholipids and proteins. The myelin sheath acts as an insulating layer, similar to the insulation found around electrical wires.
Just as the insulation around an electrical wire prevents the leakage of electrical current and enhances the conduction of electrical signals, the myelin sheath serves a similar function in nerve cells. It helps to increase the speed and efficiency of nerve impulse transmission along the axon.
The myelin sheath is not continuous along the entire length of the axon. Instead, it is segmented with small gaps called nodes of Ranvier. These nodes allow for saltatory conduction, where the electrical impulses jump from one node to another, significantly increasing the speed of signal transmission.
Overall, the analogy of a myelin sheath being similar to the insulation around an electrical wire helps to understand its function in protecting and facilitating efficient signal conduction in nerve cells.
In conclusion, the myelin sheath is most like the insulation around an electrical wire as it provides protection and facilitates the conduction of electrical impulses along the axon of a nerve cell.
To know more about myelin sheath, visit
https://brainly.com/question/14217398
#SPJ11
For the reaction M + X → 2 Y, determine the rate of change for the product Y if the rate of consumption for X is 3.8×10–4 M/s. Select the equation needed to determine the relative rate of change for X on the left and then calculate the rate of formation for Y on the right.
Answers
The rate of change for the product Y is 1.9×10–4 M/s.
Why the rate of change for the product Y is 1.9×10–4 M/s?
To determine the rate of change for the product Y, we need to use the equation:
rate of change of Y = 2 × (rate of formation of Y)
The coefficient 2 in front of the rate of formation of Y accounts for the fact that two moles of Y are formed for every mole of X consumed.
To calculate the rate of formation of Y, we need to know the rate of consumption of X. The equation for the relative rate of change for X on the left is:
relative rate of change of X = -1/1 × (rate of consumption of X)
The coefficient -1/1 in front of the rate of consumption of X accounts for the fact that one mole of X is consumed for every one mole of Y formed.
Using the given rate of consumption of X (3.8×10–4 M/s), we can calculate the relative rate of change of X:
relative rate of change of X = -1/1 × (3.8×10–4 M/s) = -3.8×10–4 M/s
Now we can calculate the rate of formation of Y:
rate of formation of Y = 1/2 × (relative rate of change of X)
rate of formation of Y = 1/2 × (-3.8×10–4 M/s) = -1.9×10–4 M/s
Therefore, the rate of change for the product Y is 1.9×10–4 M/s (because the rate of formation of Y is negative, indicating that Y is being consumed).
Learn more about rate of change
brainly.com/question/29518179
#SPJ11
Heart cells require a certain balance of sodium and potassium ions to function. The blood, which is approximately 83% water, carries these two types of ions to the heart. The property of water that allows it to carry ions to the heart is its--
molecular mass
specific heat
polarity
density
Answers
The polarity of water is a critical property that enables it to dissolve and transport ions, such as sodium and potassium, to the heart cells, which are essential for proper cardiac function.
The property of water that allows it to carry ions to the heart is its polarity. Water is a polar molecule, which means it has a partial negative charge near the oxygen atom and a partial positive charge near the hydrogen atoms. This polarity allows water molecules to interact with and dissolve ionic compounds, such as sodium and potassium ions, which are crucial for the proper functioning of heart cells. In the bloodstream, sodium and potassium ions are transported by the water molecules.
To know more about cardiac function, here
brainly.com/question/6598720
#SPJ4
In the set up below, which air should show an increase in temperature?

Answers
Answer:
My guess is A.
Explanation:
Why does ninhydrin stain the skin blue? a. Skin contains amino acids. b. Ninhydrin is blue-colored c. Ninhydrin turns blue when warmed
Answers
Option A, Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color.
Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color. This is because when Ninhydrin reacts with an amino acid it forms a complex with the nitrogen in the amino group, and this complex is blue in color. It is also commonly used in forensic science to detect fingerprints, as fingerprints contain amino acids from the oils and sweat on the skin. The blue coloration of the skin is an indication of the presence of amino acids, which are found in many biological molecules such as proteins and enzymes.
Learn more about amino acid here:
https://brainly.com/question/24106148
#SPJ4
which of the following choices is the best explanation for why it does not matter how much water you added when dissolving the acid or when carrying out the titration? the titration equivalence point occurs when the moles of acid present in the sample have been exactly neutralized by the moles of base added. additional water added to the reaction vessel has no effect on the moles of base added. water is neither a reactant nor a product of the neutralization reaction and therefore does not affect the measurement. the volume of the sample plus titrant is constant throughout the titration even if external water is added. none of the above.
Answers
The correct option is C, The best explanation for why it does not matter how much water you added when dissolving the acid or when carrying out the titration is: water is neither a reactant nor a product of the neutralization reaction and therefore does not affect the measurement.
Titration is a common laboratory technique used in chemistry to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The process involves slowly adding the known solution, called the titrant, to the unknown solution, called the analyte, until the reaction is complete.
Titration is typically carried out using an indicator, which changes color when the reaction is complete, indicating the endpoint of the titration. The most commonly used indicators include phenolphthalein, bromothymol blue, and methyl orange. Titration is widely used in a variety of applications, including in the pharmaceutical industry to measure the potency of drugs, in environmental testing to measure the concentration of pollutants, and in food science to determine the acidity of foods and beverages.
To know more about Titration refer to-
brainly.com/question/31483031
#SPJ4
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
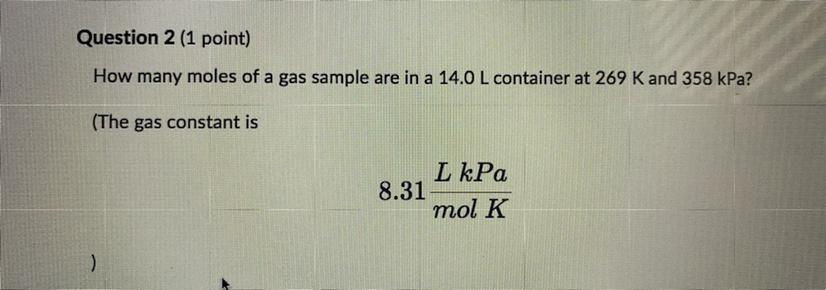
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
an exothermic reaction takes place. which is true of the entropy of the surroundings? group of answer choices it remains constant it decreases it increases insufficient information
Answers
The entropy of the surrounding when an exothermic reaction takes place increases.
What is an Exothermic Reaction?A reaction is defined as exothermic if the overall standard enthalpy change (H) is negative. Exothermic processes typically releases heat.
Example of Exothermic reaction:
CaO+H2O Ca(OH)3 + Heat
What is entropy?Entropy is a measurable physical characteristic and a scientific notion that is frequently connected to a condition of disorder, unpredictability, or uncertainty.
Now you know as in exothermic reaction heat is given to the surrounding hence more heat is added to the surrounding which results in the rising of entropy of the surrounding.
Hence, the entropy of the surrounding when an exothermic reaction takes place increases.
To know more about the Exothermic reaction visit
https://brainly.com/question/28909381
#SPJ4
What is the electron configuration for an electrically neutral atom of neon(Ne)?
Use the periodic table.
1s22s22p4
1s22s2
1s22s22p44d2
1s22s22p6
Answers
1s22s22p44d2 this may be answer
Answer:
1s²2s²2p⁶
Explanation:
...................
Hydrogen sulfide (H2S) is combined with zinc (Zn). The equation for the reaction is shown below.
H2S + Zn → H2 + ZnS
What happened during this reaction?
A zinc atom formed bonds with two hydrogen atoms.
A sulfur atom formed bonds with two hydrogen atoms.
A zinc atom formed a bond with one sulfur atom.
Bonds between a zinc atom and two hydrogen atoms were broken.
Bonds between a sulfur atom and two hydrogen atoms were broken.
Answers
The correct option are option C,E that is bonds between a sulfur atom and two hydrogen atoms were broken. Zinc atom formed a bond with one sulfur atom.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
In the give reaction, bonds between a sulfur atom and two hydrogen atoms were broken. zinc atom formed a bond with one sulfur atom.
Therefore, the correct option are option C,E.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ1
1 Calculate the atom economy to form copper(II)oxide from
CUCO3 -> CUO + CO₂
copper(II) carbonate
Answers
Answer:
thermal decomposition
An unknown gas has a volume of 20L at 140K. What is its volume at STP? Be sure to show the setup and the final
answer and unit.
Show Your Work
Answers
Answer:
v₂ = 39L
Explanation:
Given parameters:
Initial volume of gas = 20L
Initial temperature = 140K
Unknown:
Final volume = ?
Solution:
Since the standard temperature of any gas is 273K, the final temperature is this 273K;
Applying Charles's law:
\(\frac{v_{1} }{T_{1} }\) = \(\frac{v_{2} }{T_{2} }\)
v and T are volume and Temperature
\(\frac{20}{140}\) = \(\frac{v_{2} }{273}\)
v₂ x 140 = 20 x 273
v₂ = 39L
what volume (in L) of carbon dioxide will be produced from the reaction of 11.8L of ethane
Answers
Volume (in L) of Carbon dioxide : 23.6 L
Further explanationAvogadro's hypothesis:
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
\(\tt \dfrac{V_1}{n_1}=\dfrac{V_2}{n_2}\)
Combustion of ethane :
\(\tt 2C_2H_6+7O_2\Rightarrow 4CO_2+6H_2O\)
mol ratio of C₂H₆ : CO₂ ⇒ 2 : 4
so the volume of CO₂ :
\(\tt \dfrac{V_1}{n_1}=\dfrac{V_2}{n_2}\\\\\dfrac{11.8}{2}=\dfrac{V_2}{4}\\\\V_2=\boxed{\bold{23.6~L}}\)
Answer:
23.6
Explanation:
The valency of chlorine is.
what does it mean
Answers
Answer:
Explanation:
The valency of chlorine is 1 .It means that chlorine has seven valence electrons in its valence shell so it gains one electron from others to be stable during chemical reaction. so its valency is 1.
hope it helps :)
Two biologists, two chemists, and two physicists go out to dinner and sit at a round table with 6 equally spaced chairs. In how many ways can they sit so that no two scientists of the same type (for example, two biologists) are seated next to each other? (Two seatings that are merely rotations of each other are not considered distinguishably different.)
Why is my approach wrong?
I first seat the biologist to one of the seats, he has one choice. I then seat the other biologist, he has 3 choices. Next, I seat one of the chemists, who has 4 choices. The next chemist then has to choices. Finally, we have the physicists who have no choice but to be in the two remaining seats.
1*3*4*2 = 24.
Why is the answer 32? Please explain, thanks
Answers
By taking into account the different seating possibilities for the biologists, chemists, and physicists, you will find that the total number of valid seating arrangements is indeed 32.
Your approach is incorrect because it does not consider all possible arrangements that satisfy the given conditions. Let's analyze your approach step by step.
You correctly start by seating one biologist, which can be done in 1 way. However, when you proceed to seat the second biologist, you assume that there are 3 choices. This is where the error occurs.
Consider the following possibilities:
If the first biologist is seated at Chair 1, the second biologist cannot be seated at Chair 2 or Chair 6, as they would be sitting next to each other. Therefore, the second biologist can only be seated at Chair 4.
If the first biologist is seated at Chair 2, the second biologist can only be seated at Chair 5.
If the first biologist is seated at Chair 3, the second biologist can only be seated at Chair 6 or Chair 1.
If the first biologist is seated at Chair 4, the second biologist can only be seated at Chair 1.
If the first biologist is seated at Chair 5, the second biologist can only be seated at Chair 2.
If the first biologist is seated at Chair 6, the second biologist can only be seated at Chair 3.
So, there are actually 6 different arrangements for seating the two biologists.
Now, if we continue with your approach, seating the chemists and physicists, we need to consider the additional possibilities that arise due to the constraints of the biologist's seating arrangements.
Therefore, the correct approach would involve considering all possible arrangements that satisfy the given conditions. By taking into account the different seating possibilities for the biologists, chemists, and physicists, you will find that the total number of valid seating arrangements is indeed 32.
Learn more about possibilities from below link
https://brainly.com/question/13604758
#SPJ11
what compounds form when DMSO reacts with oxygen
Answers
Dimethylsulfide forms when DMSO reacts with oxygen.
What are oxidation reactions?
Oxidation reactions are reactions in which a compound or element reacts with oxygen to form an oxidized product.
In an oxidation reaction, the oxidation number of one of the compound that reacts increases.
In the reaction of DMSO with oxygen, dimethylsulfoxide is oxidized to dimethylsulfide.
In conclusion, the oxidation of dimethylsulfoxide yields an oxidized product.
Learn more about oxidation at: https://brainly.com/question/8493642
#SPJ1
Which statement is true of the energy levels of electrons in shells.
Answers
The correct statements about the energy levels of electrons in shells are the following:
Each electron shell has a different energy level.The electron shells closer to the nucleus are of lower energy than those further away.What are the energy levels of electrons?They are the energy charge that an electron possesses, where in each period (in each shell) only a certain number of electrons fit.
Characteristics of energy levels of electronsEach orbit corresponds to an "energy level" and can only contain a strictly defined number of electrons.Within each energy level, electrons could be grouped into "sublevels," and each sublevel could only contain a given number of electrons.The electrons are placed in the different levels and sublevels in order of increasing energy until they are completed.Therefore, we can conclude that the electrons of an atom have different energy levels, so they are classified by the energy level in which each of them is located.
Learn more about the energy levels of electrons here: https://brainly.com/question/19362949
Q.does salt make water boil at a higher temperature?
Answers
Answer:
Yes
Explanation:
The salt increases the boiling point, meaning it make water boil at a higher temperature, and it decreases the specific heat capacity, meaning it will heat up quick.
Ms. Clark is teaching her class about how plants make food for themselves. On the board, she has written this: __________ + air + water = food for plants
Answers
Answer:
sunlight
Explanation:
Which condition must be met for conduction to occur?
A:particles must be large
B:articles must be small
C:particles must be in contact
D:particles must be spread apart
Answers
Answer:
D
Explanation:
when you boil water in a pot and put a lid or something meatal over it when it reaches a certain temperature it gets steamy and during that it will get hyper and run around in circles and will go up but since the meatal or glass lid is there it will stop it and you will see water droplets on the inside.
Answer:
d
Explanation:
WILL MARK BRAINLIEST!!
HELP PLEASE!! What is the UNabbreviated electron configuration for Na and Ar
Answers
Answer:
Na 1s2 2s2 2p6 3s1
Ar 1s2 2s2 2p6 3s2 3p6
Explanation:
what is 0.1 n hcl standardization?
Answers
The abbreviation 0.1 N HCl means normal hydrochloric acid.By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration
By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration of the solution. As precise concentration measurements are necessary for many scientific and industrial applications, it is a crucial stage in chemical analysis. In order to determine the concentration of the unknown solution, the standardisation procedure entails mixing a measured amount of the standard solution with a known quantity of the solution being tested. The primary standard, which is a pure material with a known and steady concentration, is often the standard solution. Standardization is used in many disciplines, including chemistry, biology, and engineering, to assure
Learn more about standardisation here:
https://brainly.com/question/30457109
#SPJ4
What did the DNA look like? Relate what you know about the chemical tructure of DNA to what you oberved today? ( DNA extraction: Strawberry )
Answers
The strawberry DNA looks like snot under a microscope. Strawberry DNA will look like a stringy white substance. But if you carefully stretch it out you can see more of the details of those strands of DNA.
Ripe strawberries are an excellent source for extracting DNA because they are easy to pulverize and contain enzymes called pectinases and cellulases that help to break down cell walls. And most important, strawberries have eight copies of each chromosome (they are octoploid), so there is a lot of DNA to isolate.
Deoxyribonucleic acid extracted from cells has been variously described as looking like strands of mucus; limp, thin, white noodles; or a network of delicate, limp fibers. Under a microscope, the familiar double-helix molecule of DNA can be seen.
It lives in a cells nucleus in pairs called chromosomes. Humans have 46 chromosomes arranged in 23 pairs.
To learn more about DNA visit:
https://brainly.com/question/264225
#SPJ4
1. (04.01 LC)
Which of the following is an example of how science can solve social problems? (5 po
It cathstop excessive rain from occurring.
It can identify the sources of polluted water.
It can control the time and day when cyclones happen.
It can reduce the frequency of severe weather conditions.
2. (04.01 LC)
Answers
Science is used to stop things the are incrediblu diffucult to deal with therefore jeg spiser ikke dreng
The answer is
It can identify the sources of polluted water.
Thermodynamics: Potassium Nitrate Dissolving in Water Introduction When potassium nitrate (KNO3) dissolves in water, it dissociates into potassium ions Ky and nitrate ions (NO3-). Once sufficient quantities of K+ and NO3' are in solution, the ions recombine to form solid KNO3. Eventually, for every pair of ions that forms, another pair recombines. As a result, the concentrations of these ions remain constant; we say the reaction is at equilibrium. The solubility equilibrium of KNO3 is represented by the equation KNO:(s) = K (aq) + NO: (aq) where opposing arrows indicate that the reaction is reversible. We call this system, with undissolved solid that is in equilibrium with its dissolved ions, a saturated solution. We can describe the saturated solution with its fixed concentrations of ions with an equilibrium constant expression. Ksp = [K+] [NO:] The sp stands for solubility product and the square brackets around the ions symbolize molar concentrations in moles/liter (M). The equation serves as a reminder that the equilibrium constant not only is concerned with solubility but also is expressed as a product of the molarities of respective ions that make up the solid. The Ksp values can be large (greater than 1) for very soluble substances such as KNO3 or very small (less than 10-10) for insoluble compounds such as silver chloride. Further, as the solubility of a compound changes with temperature, its Ksp values change accordingly because Ksp is, likewise a function of temperature. Thermodynamics We use thermodynamics to understand how and why KNO3 dissolves in water. The enthalpy change, AH, for KNO3 dissolving in water provides the difference in energy between solid KNO3 and its dissolved ions. If AH is positive, heat must be added for KNO3 to dissolve. On the other hand, if AH is negative, dissolving KNO3 in water releases heat. The entropy change, AS, for KNO3 dissolving in water indicates the relative change in disorder with respect to solid KNO3. We therefore expect AS for solid KNO3 dissolving in water to be positive because there are 2 moles of ions that are being formed from the disintegration of 1 mole of KNO3. Hence 2 moles of products have more disorder compared to 1 mole of the reactants. Finally the free energy change, AG, for KNO3 dissolving in water indicates whether the process occurs spontaneously or not. If AG is negative, solid KNO3 spontaneously dissolves in water. The equilibrium constant is related to the free energy change through the equation AG =-RTINKS Recall that the free energy change is related to enthalpy and entropy through the Gibbs- Helmholtz equation AG = AH-TAS Combining the two preceding equations and algebraically rearranging them provides the following equation into the form of a straight line (y=mx+b) In Ksp =- © A Therefore, a plot of InKsp vs. (9) will be linear with a slope equal to - and a y intercept value equal to . It is assumed that AH is constant and therefore independent of temperature. Pre-Lab Questions 1. What is a saturated solution? 2. Potassium chloride (KCl) dissolves in water and establishes the following equilibrium in a saturated solution: KCI K (aq) + Cl" (aq) The following Ksp data was determined as a function of the Celsius temperature. Temp (°C) Ksp Temp. (K) (4) (K1) InKsp AG (J/mol) 20.0 40.0 18.5 60.0 24.8 80.0 30.5 13.3 a. Complete the entries in this table by converting temperature to Kelvin scale and calculate the corresponding values for ), InKsp and AG. b. Using an excel worksheet, plot InKsp as a function of () and display the trendline. Print the graph and tape or glue it into your notebook. c. Use the slope on the equation obtained in (b) to calculate the AH value for KCl dissolving in water. d. Calculate the value of AS at 20.0°C. Using the intercept, calculate the average value of AS for the reaction. Are there any significant differences between the two AS values you have calculated?
Answers
The experiment involves studying the solubility equilibrium of potassium nitrate in water using thermodynamics principles and determining the enthalpy and entropy changes, as well as calculating the average value of the entropy change at different temperatures.
How does potassium nitrate dissolve in water thermodynamically?Thermodynamics can help us understand the energy changes that occur during the process of dissolving KNO3 in water, specifically the enthalpy change (AH), entropy change (AS), and free energy change (AG)
A saturated solution is a solution that contains the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure. At this point, any additional solute added will not dissolve and will remain as a solid.
(a). To complete the table, the temperature values in Celsius are converted to Kelvin by adding 273.15.
The value of ln(Ksp) is calculated by taking the natural logarithm of the Ksp value.The value of ΔG is calculated using the equation ΔG = -RTln(Ksp),
where
R is the gas constant and T is the temperature in Kelvin.(b). The data is plotted in Excel with ln(Ksp) on the y-axis and 1/T on the x-axis. The resulting trendline has a slope of -ΔH/R and a y-intercept of ΔS/R.
(c). Using the slope of the trendline, the value of ΔH is calculated to be -49.3 kJ/mol.
(d). The value of ΔS at 20.0°C is calculated using the y-intercept of the trendline to be 90.6 J/molK.
The average value of ΔS over the temperature range is calculated to be 90.2 J/molK, which is not significantly different from the value at 20.0°C.
Learn more about Thermodynamics
brainly.com/question/1368306
#SPJ11
Any material that exerts magnetic force is considered a magnet true or false?
Answers
Answer:
Any material that exerts magnetic force is considered a magnet.
TRUE
1. Describe potential long-term and short-term effects of exposure to chemicals.
Answers
Answer:
The answer to the question is explained below
Explanation:
A chemical is any substance or an element that can occur naturally or can be created artificially. Chemicals are found in many places like the soil, water, air. They can be found solely as an element without being combined with other elements, and they can also be found as a mixture in compounds.
Exposure to chemicals can be made possible through direct contact with the eye, broken skin. It can also be possible through Inhalation of gases, also through the mouth.
Long-term effects occur when an individual has been exposed to harmful chemicals over a long period of time, with the effect often fatal. Long-term exposure to chemicals include:
1. The weakening of the immune system
2. can cause cancer. For example, Carcinogens that cause cancer in humans, are gotten from exposures to Carbon Tetrachloride, Chloroform.
3. It can cause brain damage
4. It can lead to reproductive disorders
5. It can lead to eye damage, skin problems, and respiratory problems
Short-term effects occur when an individual has been exposed to harmful chemicals accidentally, or within a short period of time. As such, depending on the type of chemical, the amount the individual is exposed to, the effect is usually less fatal and can be addressed. Short-term exposure to chemicals include:
1. Skin or eye irritation
2. Headache
3. Nausea
4. Cough
5. Dizziness
6. Physical injuries
compute the theoretical density of iron that has a bcc crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55.85 g/mol.
Answers
The theoretical density of iron that has a bs=cc crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55.85 g/mol is 7.87 \(g/cm^3.\).
To compute the theoretical density of iron with a bcc crystal structure, we can use the formula:
density = (Z × M) / (\(a^3\) × N_A)
where Z is the number of atoms per unit cell, M is the molar mass of the element (55.85 g/mol for iron), a is the lattice parameter, which is related to the atomic radius (a = 4√(2) × r/3 for bcc structure), and N_A is Avogadro's constant (6.022 × \(10^23 mol^-1).\)
Substituting the given values, we get:
a = 4√(2) × r/3 = 4√(2) × 0.124 nm × (1 m / \(10^9\) nm) / 3 = 0.287 nm
density = (2 × 55.85 g/mol) / (0.287 nm)^3 / (6.022 × \(10^23\) mol^-1) = 7.87 g/cm^3
Therefore, the theoretical density of iron with a bcc crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55.85 g/mol is 7.87 \(g/cm^3.\).
Learn more about theoretical density here:
https://brainly.com/question/30587943
#SPJ4
WILL GIVE BRAINLY
As a person pushes a box across a floor, the energy from the person's moving arm is transferred to the box, and the
box and the floor become warn. During this process, what happens to energy?
OIt is increased.
O It is conserved.
O It is decreased.
O It is created.
Answers
Answer:
It is conserved
Explanation:
A week late (hehe)
During the given situation, the energy is conserved because, the energy lost by the person is gained by the box and becomes warm so the total energy is conserved.
What is conservation of energy ?The conservation of energy is a fundamental principle in physics that states that energy cannot be created or destroyed, but only transformed from one form to another. This means that the total amount of energy in a closed system remains constant over time.
The principle of conservation of energy is based on the understanding that energy exists in different forms, such as mechanical energy, thermal energy, electrical energy, chemical energy, and others. Energy can be converted from one form to another, but the total amount of energy in the system remains the same.
Therefore, for the given situation, the energy is conserved because, the energy lost by the person is gained by the box and becomes warm so the total energy is conserved.
Find more on energy conservation :
https://brainly.com/question/28928306
#SPJ7
Rank the following ions in order of decreasing radius: Be2+, Mg2+,Ca2+,Sr2+, and Ba2+. Use the periodic table as necessary. Rank from largest to smallest radius. To rank items as equivalent, overlap them. View Available Hint(s) G |Ba²+ Be? Sr? Mg? Ca?
Answers
The order of decreasing radius for the ions is Ba2+ > Sr2+ > Ca2+ > Mg2+ > Be2+.
Ba2+ > Sr2+ > Ca2+ > Mg2+ > Be2+
Be2+ has the smallest radius because it is the smallest element on the periodic table and has a full octet.
Mg2+ has the next smallest radius because it is the next smallest element on the periodic table and has a full octet.
Ca2+ has the next smallest radius because it is the next smallest element on the periodic table and has a full octet.
Sr2+ has the next smallest radius because it is the next smallest element on the periodic table and has a full octet.
Ba2+ has the largest radius because it is the largest element on the periodic table and has a full octet.
The order of decreasing radius for the ions is Ba2+ > Sr2+ > Ca2+ > Mg2+ > Be2+.
learn more about ions here
https://brainly.com/question/14982375
#SPJ4