Answers
Answer:
C
Explanation:
Have a nice day
Related Questions
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
What is the change in temperature if 500 J of heat are added to a 23 g sample of silver? (Thespecific heat of silver is 0.24 J/g °C)
Answers
The change in temperature (∆T) if 500 J of heat are added to a 23 g sample of silver is calculated to be 90.58°C.
How to calculate change in temperature?The change in temperature of a substance can be calculated using the following formula:
Q = mc∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substancec = specific heat capacity∆T = change in temperature500 = 23 × 0.24 × ∆T
500 = 5.52∆T
∆T = 500/5.52
∆T = 90.58°C
Therefore, the change in temperature (∆T) if 500 J of heat are added to a 23 g sample of silver is calculated to be 90.58°C.
Learn more about change in temperature at: https://brainly.com/question/19051558
#SPJ1
I need help with this fill in the blank question


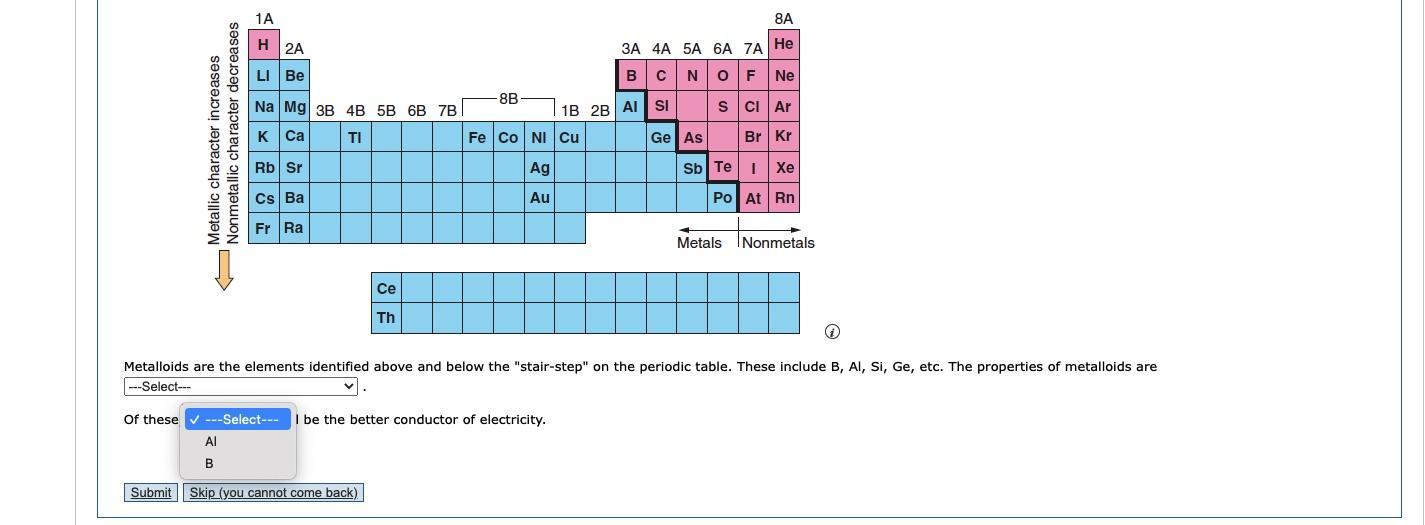
Answers
Answer: Most metalloids have some physical properties of metals and some of non-metals. So metalloids are similar to metals and non-metals.
Aluminium (Al) is a better conductor of electricity than Boron (B) which is a non-metal.
What do atoms make up
Answers
17. HAZWOPER training and certification recognizes:
a. A large number (as much as 80%) will self-present or be self-referred victims
b. Awareness level training will promote proper initial triage actions
c.
Victims will use any entrance they can enter at the hospital, in addition to the
emergency department entrance
d. Both A and C
Answers
HAZWOPER training and certification recognize:
a large number (as much as 80%) will self-present or be self-referred victimsVictims will use any entrance they can enter at the hospital, in addition to the emergency department entranceThe correct option is both A and C
What is the HAZWOPER training and certification?HAZWOPER (Hazardous Waste Operations and Emergency Response) training and certification recognize that a large number of victims (as much as 80%) in hazardous waste incidents or emergencies will self-present or be self-referred for medical treatment.
Additionally, HAZWOPER training acknowledges that victims may use any entrance they can access at a hospital, not just the emergency department entrance.
This is because individuals affected by hazardous materials may arrive at different areas of the hospital seeking medical assistance.
Therefore, option d. Both A and C are correct statements regarding the recognition of HAZWOPER training and certification.
Learn more about HAZWOPER at: https://brainly.com/question/31561828
#SPJ1
Write the chemical formula for this molecule

Answers
The chemical formula for the molecule you provided is C2H5Cl.
In the molecule, the central atom is carbon (C), which is bonded to two hydrogen atoms (H) and one chlorine atom (Cl). The carbon atom forms single bonds with each of the hydrogen and chlorine atoms, resulting in a linear structure.
To write the chemical formula, we start by indicating the number of atoms of each element present in the molecule. In this case, there are two carbon atoms (C2), five hydrogen atoms (H5), and one chlorine atom (Cl1).
Next, we write the symbols for the elements in the order of their appearance. The formula is typically written with the carbon atom first, followed by hydrogen, and then any other elements in alphabetical order. Therefore, the chemical formula for the molecule is C2H5Cl.
The subscripts in the formula indicate the number of atoms of each element in the molecule. In this case, there are two carbon atoms, five hydrogen atoms, and one chlorine atom.
It's important to note that the formula represents the simplest ratio of atoms in the molecule. It does not provide information about the spatial arrangement or bonding pattern of the atoms. Additional structural information, such as the arrangement of atoms in space, would require a more detailed representation, such as a Lewis structure or a three-dimensional model.
for more questions on chemical formula
https://brainly.com/question/21393201
#SPJ8
Why are covalent substances gases and liquid rather than solids?
Answers
Covalent compounds are held together with an intra molecular attraction which is weaker than metallic bond
hence covalent compounds exist as liquids, gases and soft solids
A solution contains 3.5 mol NaCl and 4.2 mol MgCl₂. How many equivalents of chloride ion are present?
Answers
There are 15.4 equivalents of chloride ion present in the solution
To calculate the number of equivalents per mole of chloride ionWe need to multiply the total number of moles of chloride ion in the solution by the number of equivalents.
The molar mass of NaCl is 58.44 g/mol, so 3.5 mol of NaCl contains :
3.5 mol NaCl x 2 mol Cl⁻/1 mol NaCl = 7 mol Cl⁻
Similarly, the molar mass of MgCl₂ is 95.21 g/mol, so 4.2 mol of MgCl₂ contains:
4.2 mol MgCl₂ x 2 mol Cl⁻/1 mol MgCl₂ = 8.4 mol Cl⁻
Therefore, the total number of moles of chloride ion in the solution is:
7 mol Cl⁻ + 8.4 mol Cl⁻ = 15.4 mol Cl⁻
By dividing the total number of moles by the number of equivalents per mole, we can finally determine how many equivalents of the chloride ion there are. There is one equivalent of the chloride ion per mole since it has a valency of -1.
15.4 mol Cl⁻ x 1 eq/mol = 15.4 eq
So there are 15.4 equivalents of chloride ion present in the solution.
Learn more about mole here : brainly.com/question/30337257
#SPJ1
An atom has a mass number of 9 and 5 neutrons. What is its atomic number? A 19 B. 14 O c.4 D. 11 SN
Answers
Answer:
\(\boxed {\boxed {\sf C. \ 4}}\)
Explanation:
First, define some important terms.
Mass number: the sum of the nucleons (protons and neutrons) Atomic number: the number of protonsWe know the mass number is 9. So, the sum of protons and neutrons is 9.
\(mass \ number =9\)\(protons + neutrons = 9\)We also know the atoms has 5 neutrons. Therefore, the rest must be protons.
\(protons + 5 \ neutrons =9\)Subtract the 5 neutrons from the overall mass number of 9.
\(protons = 9- 5 \ neutrons \\\)\(protons=4\)There are 4 protons, so the atomic number is also 4. This atom is beryllium.
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
What volume would 0.435 moles of hydrogen gas, Hz, occupy at STP?
Answers
Answer:
will be 9.7 Liters
Explanation:
Fill in the coefficients that will balance the following reaction: (Note: Use 1 as coefficient where appropriate.) NaCl + CaS → Na2S + CaCl2
Answers
Answer:
the answer is
2,1,1,1
Explanation:
Macmillan Learning
Calculate the standard change in Gibbs free energy for the reaction at 25 °C. Standard Gibbs free energy of formation values can
be found in this table.
Fe₂O3(s) + 2Al(s)
AG=
先
Bi
B
1
Al₂O₂ (s) + 2 Fe(s)
45°F Cloudy
kJ/mol
4 ENG
9:05 PM
3/23/2003
48
4
+
B
*
Answers
The standard change in Gibbs free energy for the reaction at 25 °C is 278.0 kJ/mol for the given enthalpy of reaction .
What is Gibbs free energy ?The Gibbs free energy (or Gibbs energy as the preferred name; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of non-volume expansion work that a thermodynamically closed system can perform at constant temperature and pressure. It also serves as a prerequisite for processes like chemical reactions that may place under these conditions. The Gibbs free energy is denoted by the symbol G(p,T) = U+pV-TS = H-TS, where p denotes pressure, T denotes temperature, U denotes internal energy, V denotes volume, H denotes enthalpy, and S denotes entropy.
What is enthalpy of reaction ?A thermodynamic quantity equal to a system's entire heat content. It is equivalent to the system's internal energy plus the product of pressure and volume.
According to the table, the standard Gibbs free energy of formation values are;
Fe₂O₃ (s) = -822.1 kJ/mol
Al₂O₃ (s) = -1675.2 kJ/mol
Al (s) = -1477.7 kJ/mol
Fe (s) = 0 kJ/mol
The reaction is:
Fe₂O₃ (s) + 2 Al (s) → Al₂O₃ (s) + 2 Fe (s).
Therefore, the standard change in Gibbs free energy for the reaction at 25 °C is:
AG = -822.1 kJ/mol + (2 x -1477.7 kJ/mol) - (-1675.2 kJ/mol) - (2 x 0 kJ/mol) = 278.0 kJ/mol
To know more about Gibbs free energy ,visit ;
https://brainly.com/question/20358734
#SPJ1
Explain how the elimination of a predator from an ecosystem might result in starvation amongst its prey species.
Answers
Answer:
If the primary predator of a prey species is eliminated, the prey species’ population will increase to the point of overpopulating the ecosystem. This overpopulation will result in depletion of the prey species’ food supplies as more individuals compete for the same resources and can ultimately lead to the prey species starving.
Explanation:
Elimination of a predator from an ecosystem might lead to famine amongst its prey species because:
the populations of the prey species thrive in the absence of selective pressure by the predatorthe thriving of the populations will put pressure on the available food resources for the prey species leading to significant intra-species competition.available foods might eventually get depleted as population and competition surge.food depletion means the population may end up dying of hunger if the status quo remains.More on food webs can be found here: https://brainly.com/question/2233704?referrer=searchResults
2. Molten Iron metal and carbon monoxide are produced in a blast furnace by the reactionof iron(III) oxide and pure carbon. If 70.0 grams of Fe203 are used, how many grams ofiron can be produced?The Balanced reaction is:

Answers
Answer
The Balanced reaction is: Fe₂O₃ + 3C → 2Fe + 3CO
The grams of iron (Fe) produced = 48.96 grams.
Explanation
Given:
Mass of Fe₂O₃ used = 70.0 grams
What to find:
The grams of iron (Fe) produced.
Step-by-step solution:
The balanced reaction between iron(III) oxide and pure carbon in a blast furnace to produce molten iron metal and carbon monoxide is:
Fe₂O₃ + 3C → 2Fe + 3CO
The next step is to convert 70.0 grams of Fe₂O₃ to moles using the mole formula
\(\begin{gathered} Moles=\frac{Mass}{Molar\text{ }mass} \\ \\ The\text{ }molar\text{ }mass\text{ }of\text{ }Fe₂O₃=159.69\text{ }g\text{/}mol \\ \\ Moles=\frac{70.0\text{ }g}{159.69\text{ }g\text{/}mol} \\ \\ Moles=0.438349301\text{ }mol\text{ }Fe₂O₃ \end{gathered}\)Now, we can use the moles of Fe₂O₃ and the mole ration from the equation above to determine the moles of Fe produced.
\(\begin{gathered} 1\text{ }mol\text{ }Fe₂O₃=2\text{ }mol\text{ }Fe \\ \\ 0.438349301\text{ }mol\text{ }Fe₂O₃=x\text{ }mol\text{ }Fe \\ \\ x=\frac{0.438349301\text{ }mol\text{ }Fe₂O₃}{1\text{ }mol\text{ }Fe₂O₃}\times2\text{ }mol\text{ }Fe \\ \\ x=0.876698603\text{ }mol\text{ }Fe \end{gathered}\)Finally, we can convert 0.876698603 mol Fe to mass in grams as follows
\(\begin{gathered} Mass=Moles\times Molar\text{ }mass \\ \\ Molar\text{ }mass\text{ }of\text{ }Fe=55.845\text{ }g\text{/}mol \\ \\ Mass=0.876698603\text{ }mol\times55.845\text{ }g\text{/}mol \\ \\ Mass=48.96\text{ }g \end{gathered}\)The grams of iron (Fe) produced is 48.96 grams.
Assignment Tools
r
A⟶products
()
(−1)
275
0.379
725
0.676
What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values.
1=
1=
2=
2=
Determine the rise, run, and slope of the line formed by these points.
rise:
run:
slope:
What is the activation energy of this reaction?
a=
J/mol
Hi. Can you please work this problem out step by step, including the maths. In full detail.
Answers
The activation energy of this reaction is approximately -13.770 J/mol.
1. To graphically determine the activation energy, we need to plot two points. The given data points are:
Point 1: (1, -1.275)
Point 2: (2, 0.379725)
2. The rise is the change in the y-coordinate between the two points:
Rise = y2 - y1 = 0.379725 - (-1.275) = 1.654725
3. The run is the change in the x-coordinate between the two points:
Run = x2 - x1 = 2 - 1 = 1
4. The slope of the line formed by these two points can be calculated using the formula:
Slope = rise / run = 1.654725 / 1 = 1.654725
5. The activation energy (Ea) can be determined using the equation:
Ea = -R * slope
Here, R is the ideal gas constant, which is approximately 8.314 J/(mol·K).
6. Plugging in the values:
Ea = -8.314 * 1.654725 = -13.770 J/mol
Note that the activation energy is negative because it represents the energy difference between the reactants and the transition state (higher energy) in an exothermic reaction.
for more such question on energy
https://brainly.com/question/5650115
#SPJ8
Answer fast please?????????????

Answers
Answer:
option a. iron,copper, aluminium.
The smallest possible particle of an element is a
Answers
Answer:
An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
Explanation:
The smallest possible particle of an element is a atom. The remainder is made up of a cloud of negatively charged electrons around a positively charged nucleus made up of protons and neutrons.
What is atom?The smallest unit of matter that may be split without producing electrically charged particles is the atom. It is also the smallest piece of substance with chemical element-like characteristics. Electric forces, which link electrons towards the nucleus of atoms, cause them to be drawn to any positive charge.
Space makes up the majority of an atom. The remainder is made up of a cloud of negatively charged electrons around a positively charged nucleus made up of protons and neutrons. Compared to electrons, that are the smallest charged particles in nature, the nucleus is tiny and dense.
Therefore, the smallest possible particle of an element is a atom.
To learn more about atom, here:
brainly.com/question/29712157
#SPJ6
A gas mixture containing N2 and O2 was kept inside a 2.00 L container at a temperature of 23.0°C and a total pressure of 1.00 ATM the partial pressure of oxygen was 0.722 ATM how many grams of nitrogen are present in the gas mixture
Answers
Answer:
0.641 g of Nitrogen are present in the mixture.
Explanation:
We use the Ideal Gases Law, to solve this question.
For the mixture:
P mixture . V mixture = mol mixture . R . T
We convert the T° to K → 23°C + 273 = 296 K
R = Ideal gases constant → 0.082 L.atm/mol.K
1 atm . 2L = mol mixture . 0.082 L.atm/mol.K . 296K
2 atm.L / ( 0.082 mol /L.atm) . 296 = 0.0824 moles
We know that sum of partial pressure = 1
Partial pressure N₂ + Partial pressure O₂ = 1
1 - 0.722 atm = Partial pressure N₂ → 0.278 atm
We apply the mole fraction concept:
Partial pressure N₂ / Total pressure = Moles N₂ / Total moles
Moles N₂ = (Partial pressure N₂ / Total pressure) . Total moles
Moles N₂ = (0.278 atm / 1 atm) . 0.0824 mol → 0.0229 moles
We convert the moles to mass → 0.0229 mol . 28 g/mol = 0.641 g
641 mg
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Which of the following options correctly reflect the relationship between SI units and units containing decimal prefixes? Select all that apply. Check all that apply. O 1 millimole contains 1000 moles. 1nm-10 m; hence there are 10° nm in 1 m. O There are 1000 g in one kg. O There are 10 microseconds in 1 second.
Answers
There are 1000 g in one kg. It is a correctly reflect the relationship between SI units and units containing decimal prefixes.
What is meant by SI units?
Other coherent derived units may be expressed using a combination of the seven base units and the 22 coherent derived units with unique names and symbols.The SI offers twenty-four prefixes that, when applied to the name and symbol of a coherent unit or units, produce twenty-four additional (non-coherent) SI units for the same quantity since the sizes of coherent units will be convenient for some purposes but not for others.The coherent unit's decimal multiples and submultiples are always the non-coherent units.The SI is designed to be a dynamic system; new units and prefixes are added, and unit definitions are changed through international agreement as measurement technology develops and measurement accuracy rises.To learn more about SI units refer to
https://brainly.com/question/18779569
#SPJ4
What else is produced when sodium carbonate decomposes?
Na2Co3 - Na2O+
Answers
Answer:When a single compound breaks down into two or more compounds or elements in a chemical reaction then it is known as decomposition reaction.
The chemical symbol for sodium carbonate is .
The decomposition of sodium carbonate is:
The decomposition of sodium bicarbonate, will result in the formation of sodium oxide, and carbon dioxide, .
Hence, carbon dioxide, will produce with sodium oxide, on decomposition of .
Explanation:Na2CO3 Na2O +CO2 CO2 is the answer
What is the molar mass of Al(BrO2)
Answers
Answer:
The molar mass is 138.8843 g/mol
9.25 mol C2H6 reacts with 15.0 mol 02 according to the equation below:
262H6 + 702 —> 4C02 + 6H20
What mass of carbon dioxide forms during the reaction of 9.25 moles C2H6?
[?] g CO2

Answers
Answer:
Mass= 351.5kg
Explanation:
From the question
mole = 9.25mol.
Molar mass = C2H6 = 16×2+1×6=32+6=38molg.
Mass = ?
\(n = \frac{mass}{moler \: mass} \)
\(9.25 = \frac{m}{38} \\ = 9.25 \times 38 = m \\ = 351.5kg\)
therefore mass is 351.5kg
Match it, I’m not sure which category they go in

Answers
Answer:
Endothermic is something that needs heat added into it, while exoteric gives heat off
Explanation:
1. Endo(Melting ice)
2. Endo(heat enters the system)
3. Exothermic(making ice)
4. Endothermic( liquid to gas)
5. Exothermic(campfire)
6. exothermic(gas to a liquid)
7. Exothermic (the flame of a candle)
8. Exothermic (heat leaves the system)
9. I don’t know if photosynthesis is exo or endothermic
As part of an investigation of the population of foxes on Sunday Gill island a scientist graphed the number of foxes presented on the island over a Spam of 15 years as shown below the study began with the earlier 0 and run until the start of year 15 According to the graph during the witch year the event reduced the carrying capacity of the area
Answers
The carrying capacity of the area was reduced in the year 10 according to the graph that shows the number of foxes on the island over a span of 15 years.
The graph shows a population of foxes over a span of 15 years. The y-axis represents the number of foxes on the island, while the x-axis represents time. The study began with the earlier 0 and ran until the start of year 15. According to the graph, the carrying capacity of the area was reduced in the year 10.
In the graph, it is shown that the population of foxes on Sunday Gill island had a significant increase from year 0 to year 3. After year 3, the fox population started to decrease and then remained fairly constant until year 10. After year 10, the population of foxes on the island started to decline more rapidly until the end of the study in year 15
This decline in the population of foxes on the island is most likely due to the reduction in carrying capacity of the area. Carrying capacity refers to the maximum number of individuals that an environment can sustain. When the carrying capacity of an environment is reached, it means that the environment can no longer provide the necessary resources to sustain the population.
There are various factors that can cause a reduction in carrying capacity, such as environmental degradation, competition for resources, or a natural disaster. In this case, it is not clear what caused the reduction in carrying capacity in year 10, but it is likely that it was due to some environmental factor that impacted the availability of resources for the fox population.
For more such information on: graph
https://brainly.com/question/31305548
#SPJ8
What is the compound's systematic name?

Answers
Answer: methane
Explanation:
What are the possible values of 1 and m for
n=4 ?
Answers
Answer:
If n = 4, then the possible values of 1 and m depend on the equation or expression being used. Without more information, it is impossible to determine what the possible values of 1 and m might be. Can you please provide more context or information about the problem you are trying to solve?
What is the Atomic mass and the mass number of Carbon?
Answers
Answer:
I think the answer is 12.0107 u and 6 (6 as the atomic number) and (12.0107 u as the atomic mass).
Explanation:
I Hope this helps.
Calculate the heat needed to convert 25.0 grams of solid silver from 950.°C to liquid silver at 972°C. The specific heat of solid silver is 0.235 J/g C, for liquid silver it's 0.278 J/gºC. 3 steps
Answers
The heat required is 2347 J
What is the heat required for change of state?The heat required for a change of state depends on the substance, the amount of the substance, and the specific change of state involved.
When a substance undergoes a change of state, such as melting or boiling, heat is added or removed to cause the particles in the substance to gain or lose energy and rearrange themselves into a new physical state. The amount of heat required to effect this change is known as the heat of transformation, or the heat of fusion
We know that;
H1 = 25.0 * 0.235 * (962 - 950)
= 70.5 J
H2 = 25 g * 88 J/g
= 2200 J
H3 = 25 * 0.278 * (972 - 962)
=76. 5 J
Then we have that;
70.5 J + 2200 J + 76. 5 J
=2347 J
Learn more about heat:https://brainly.com/question/1429452
#SPJ1