which of the following conditions is/are met at the equivalence point of the titration of a monoprotic weak acid with a strong base? 1. the moles of base added from the buret equals the initial moles of weak acid. 2. the volume of base added from the buret must equal the volume of acid titrated. 3. the ph of the solution is greater than 7.00.
Answers
At the equivalence point of a titration, the number of moles of acid present in the solution equals the number of moles of base added from the buret.
At the equivalence point of a titration, the number of moles of acid present in the solution equals the number of moles of base added from the buret. Therefore, the first condition is met at the equivalence point of the titration of a monoprotic weak acid with a strong base. The second condition is not necessarily met, as the volume of base added may be less than or greater than the volume of acid titrated depending on the strength of the acid and base used. The third condition is generally not met at the equivalence point of the titration of a monoprotic weak acid with a strong base, as the resulting solution will typically have a pH greater than 7.00 due to the formation of the conjugate base of the weak acid. The pH at the equivalence point of a titration depends on the strength of the acid and base being used.
To know more about titration visit: https://brainly.com/question/31870069
#SPJ11
Related Questions
When backpacking in the wilderness, hikers often boil water to sterilize it for drinking. Suppose that you are planning a backpacking trip and will need to boil 35 L of water for your group. What volume of fuel should you bring? Assume that the fuel has an average formula of C7 H16 , 15% of the heat generated from combustion goes to heat the water (the rest is lost to the surroundings), the density of the fuel is 0.78 g>mL, the initial temperature of the water is 25.0 °C, and the standard enthalpy of formation of C7 H 16 is -224.4 kJ>mol.
Answers
Answer:
2.104 L fuel
Explanation:
Given that:
Volume of water = 35 L = 35 × 10³ mL
initial temperature of water = 25.0 ° C
The amount of heat needed to boil water at this temperature can be calculated by using the formula:
\(q_{boiling} = mc \Delta T\)
where
specific heat of water c= 4.18 J/g° C
\(q_{boiling} = 35 \times 10^{3} \times \dfrac{1.00 \ g}{1 \ mL} \times 4.18 \ J/g^0 C \times (100 - 25)^0 C\)
\(q_{boiling} = 10.9725 \times 10^6 \ J\)
Also; Assume that the fuel has an average formula of C7 H16 and 15% of the heat generated from combustion goes to heat the water;
thus the heat of combustion can be determined via the expression
\(q_{combustion} =- \dfrac{q_{boiling}}{0.15}\)
\(q_{combustion} =- \dfrac{10.9725 \times 10^6 J}{0.15}\)
\(q_{combustion} = -7.315 \times 10^{7} \ J\)
\(q_{combustion} = -7.315 \times 10^{4} \ kJ\)
For heptane; the equation for its combustion reaction can be written as:
\(C_7H_{16} + 11O_{2(g)} -----> 7CO_{2(g)}+ 8H_2O_{(g)}\)
The standard enthalpies of the products and the reactants are:
\(\Delta H _f \ CO_{2(g)} = -393.5 kJ/mol\)
\(\Delta H _f \ H_2O_{(g)} = -242 kJ/mol\)
\(\Delta H _f \ C_7H_{16 }_{(g)} = -224.4 kJ/mol\)
\(\Delta H _f \ O_{2{(g)}} = 0 kJ/mol\)
Therefore; the standard enthalpy for this combustion reaction is:
\(\Delta H ^0= \sum n_p\Delta H^0_{f(products)}- \sum n_r\Delta H^0_{f(reactants)}\)
\(\Delta H^0 =( 7 \ mol ( -393.5 \ kJ/mol) + 8 \ mol (-242 \ kJ/mol) -1 \ mol( -224.4 \ kJ/mol) - 11 \ mol (0 \ kJ/mol))\)
\(\Delta H^0 = (-2754.5 \ \ kJ - 1936 \ \ kJ+224.4 \ \ kJ+0 \ \ kJ)\)
\(\Delta H^0 = -4466.1 \ kJ\)
This simply implies that the amount of heat released from 1 mol of C7H16 = 4466.1 kJ
However the number of moles of fuel required to burn \(7.315 \times 10^{4} \ kJ\) heat released is:
\(n_{fuel} = \dfrac{q}{\Delta \ H^0}\)
\(n_{fuel} = \dfrac{-7.315 \times 10^{4} \ kJ}{-4466.1 \ kJ}\)
\(n_{fuel} = 16.38 \ mol \ of \ C_7 H_{16\)
Since number of moles = mass/molar mass
The mass of the fuel is:
\(m_{fuel } = 16.38 mol \times 100.198 \ g/mol}\)
\(m_{fuel } = 1.641 \times 10^{3} \ g\)
Given that the density of the fuel is = 0.78 g/mL
and we know that :
density = mass/volume
therefore making volume the subject of the formula in order to determine the volume of the fuel ; we have
volume of the fuel = mass of the fuel / density of the fuel
volume of the fuel = \(\dfrac{1.641 \times 10^3 \ g }{0.78 g/mL} \times \dfrac{L}{10^3 \ mL}\)
volume of the fuel = 2.104 L fuel
How are elements arranged on the periodic table?
a. they are arranged to highlight similarities between elements
b. they are arranged to highlight different elements with similar isotopes
c. they are arranged in order from most abundant to very rare and unstable
d. they are arranged in order of the date of discovery
Answers
Answer:
the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom.In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column.
The elements on the periodic table are arranged on the basis of highlighting similarities between elements. Thus, the correct option for this question is A.
How elements are arranged on the periodic table?The elements on the periodic table are basically arranged on the basis of increasing their atomic number. These atomic number is represented by the number of protons in the nucleus of an element.
According to the question, the elements possessing identical chemical properties are arranged in the same group. For example, group 17 is known as the halogen group. Among this group, the elements bear one electron short to fulfill their octet.
The whole periodic table comprises of 18 groups and 7 periods.
Therefore, the elements on the periodic table are arranged on the basis of highlighting similarities between elements. Thus, the correct option for this question is A.
To learn more about the Periodic table, refer to the link:
https://brainly.com/question/15987580
#SPJ2
Look at the activity series and select which two of the following reactions would happen on their own. (Remember, if the lone element is more active than the metal in the compound, the lone element will react and replace the metal in the compound.) Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (Al)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
A.
2Li + ZnBr2 ->2LiBr + Zn
B.
Al + 3LiCl ->AlCl3 + 3Li
C.
Sn + ZnSe ->SnSe + Zn
D.
3Ca + Al2O3 ->2Al + 3CaO
Answers
Answer:
2Li + ZnBr2 ->2LiBr + Zn
3Ca + Al2O3 ->2Al + 3CaO
Explanation:
Spontaneous reactions are reactions that can happen on their own. For a spontaneous reaction to occur, a metal that is higher in the activity series must displace a metal that is lower in the activity series from its solution and not vice versa.
If we look at the two reactions selected in the answer, lithium is above zinc in the activity series and calcium is above aluminum in the activity series hence the two reactions occur spontaneously.
You are experimenting with blood samples and you place a drop of RBCs into a solution of 300 millimoles of CaCl2. What effect does this have on the RBCs?A.) The RBCs would shrink.B.) No effect on RBCs. This is an isotonic soution and there would be no net movement of water.C.) The RBCs would swell and burst.
Answers
The drop of RBCs placed in the 300 millimolar solution of CaCl2 would cause the RBCs to shrink. The answer is A) The RBCs would shrink.
This is because the solution is hypertonic, meaning it has a higher solute concentration than the RBCs. As a result, water will move out of the RBCs to try to equalize the concentration of solutes on both sides of the membrane. This will cause the RBCs to shrink in size as they lose water. The Ca2+ ions in the solution can also interact with the negatively charged phospholipid heads in the RBC membrane, causing the membrane to become more rigid and less flexible, further contributing to the shrinkage of the RBCs.
To learn more about hypertonic Click here:
brainly.com/question/13886457
#SPJ4
What part of the atom can be used to figure out the name of the element
Answers
Answer:
number of protons
Explanation:
The number of protons determines an element's atomic number and is used to distinguish one element from another.
Select all the options that correctly describe the sublevel when referring to the Schrodinger's model of the atomA. The number of lobes of an orbital depends on the sublevel of the orbital.
B. The orbitals within a sublevel at a given principal energy level have the same energies. For example Px has the same energy as Py.
Answers
The correct statements about Schrodinger's model of the atom are option A and B: The orbitals within a sublevel have the same energies, and the number of lobes of an orbital depends on these sublevels.
In order to explain the energies of electrons in atoms and information on the shape and orientations of the most likely distribution of electrons around the nucleus, a set of values obtained from the solution of the Schrodinger equation is used to construct an orbital.
Quantum numbers are the ones in question. Principle (n), azimuthal (l), and magnetic (m) quantum numbers are the three distinct sets of quantum numbers that make up an orbital. The fourth quantum number is known as spin quantum number (s) which usually denotes the orientation of an electron inside an orbital. The values for this quantum number can either be +1/2 or -1/2.
To know more about the Schrodinger's model, refer:
https://brainly.com/question/11032278
#SPJ4
Complete question is:
Select all the options that correctly describe the sublevel when referring to the Schrodinger's model of the atom.
A. The orbitals within a sublevel at a given principal energy level have the same energies. For example px has the same energy as py.
B. The number of lobes of an orbital depends on the sublevel of the orbital.
C. The relative energies of the sublevels s, p, d, and f are (lowest energy) f < d < p < s (highest energy).
D. The number of sublevels in each principal energy level is the same.
Determine the pH of an aqueous solution made by dissolving 50 mg of H2SO4 and 60 mg of NaOH to a final volume of 500 ml at 25oC.
Answers
The pH of the aqueous solution made by dissolving 50mg of H₂SO₄ and 60 mg of NaOH to a final volume of 500 ml at 25°C is 11.10.
Step 1: Convert the mass of H₂SO₄ and NaOH to moles.
Therefore, the number of moles of H₂SO₄ present in the solution = (50 mg)/(98.08 g/mol) = 0.00051 mol.
The number of moles of NaOH present in the solution = (60 mg)/(40.00 g/mol) = 0.0015 mol
Step 2: Use the number of moles of H₂SO₄ and NaOH to calculate the limiting reactant.The limiting reactant is the chemical that's present in the solution in the smallest amount. It is the chemical that will react completely with the other chemical. The limiting reactant will, therefore, determine the number of moles of any other chemical that will react completely and the products that will be formed. H₂SO₄ is the limiting reactant since it is present in a smaller amount.
Step 3: Calculate the number of moles of H⁺ ions that will be formed when H₂SO₄ reacts completely with water.
H₂SO₄ is an acid and will react with water to form H⁺ ions and HSO₄⁻ ions. HSO4⁻ ions will also react with water to form H⁺ ions and SO4²⁻ ions.
H₂SO₄ + H₂O ⟶ H⁺ + HSO₄⁻ HSO₄⁻ + H2O ⟶ H⁺ + HSO₄²⁻
The number of moles of H⁺ ions that will be formed from 0.00051 mol of H₂SO₄ is 0.00051 mol.
Step 4: Calculate the number of moles of OH⁻ ions that will be formed when NaOH reacts completely with water.NaOH is a base and will react with water to form OH⁻ ions and Na⁺ ions.NaOH + H2O ⟶ OH⁻ + Na⁺
The number of moles of OH⁻ ions that will be formed from 0.0015 mol of NaOH is 0.0015 mol.
Step 5: Calculate the concentration of H⁺ ions and OH⁻ ions in the solution.
The concentration of H⁺ ions in the solution = (number of moles of H⁺ ions)/(volume of solution)= (0.00051 mol)/(0.5 L) = 0.00102 M
The concentration of OH⁻ ions in the solution = (number of moles of OH⁻ ions)/(volume of solution)= (0.0015 mol)/(0.5 L) = 0.003 M
Step 6: Use the equation for the ion product of water to calculate the pH of the solution.
The ion product of water is given as follows: Kw = [H⁺][OH⁻] where Kw is the ion product of water (1.0 x 10-14 at 25°C).
Hence, [H⁺][OH⁻] = Kw = 1.0 x 10-14
If [H⁺][OH⁻] = 1.0 x 10-14, then [H⁺]/[OH⁻] = 1.0 x 10-14/[OH⁻] = [H3O⁺] and pH = -log[H3O⁺].
Therefore, pH = -log[H3O⁺] = -log(1.0 x 10-14/0.003) = 11.10
The pH of the aqueous solution is 11.10.
For more questions on pH of aqueous solution: https://brainly.com/question/10951922
#SPJ11
Purchase process: (50) The process begins with a department admin sending a purchase request to the IT department. The IT Manager reviews the request and if approved, requests a quote from Apple, Dell, HP, ASUS and Lenovo. If rejected, the request is sent back to the admin for review and has to be resubmitted to the IT Manager. The best price will be sent to the admin and once approved, the IT manager finalizes the vendor and then prepares the purchase request. The Procurement Supervisor receives the request and issues the purchase order to the vendor. The Procurement Supervisor then reviews the invoice and processing time from the vendor. By the end of the processing time, if the tracking number was not received, the Supervisor cancels the order. If vendor provides the tracking number, Procurement Supervisor collects the product once delivered and simultaneously submits the payment. Once both the steps are done, the process ends as the purchase is completed.
Answers
The purchase process involves steps such as initiating a request, vendor selection, approval, purchase order issuance, product delivery, and payment, ensuring a systematic approach to procurement for accountability and efficiency.
The purchase process consists of several steps:
1. The department admin initiates the process by sending a purchase request to the IT department.
2. The IT Manager reviews the request and decides whether to approve or reject it.
3. If the request is approved, the IT Manager contacts various vendors, such as Apple, Dell, HP, ASUS, and Lenovo, to request quotes.
4. The IT Manager receives the quotes and selects the best price.
5. Once the best price is selected, the IT Manager informs the admin and waits for their approval.
6. If the admin approves, the IT Manager finalizes the vendor selection and prepares the purchase request.
7. The IT Manager then sends the purchase request to the Procurement Supervisor.
8. The Procurement Supervisor receives the request and issues a purchase order to the chosen vendor.
9. The Procurement Supervisor reviews the vendor's invoice and processing time.
10. If the processing time elapses and the tracking number has not been received, the Procurement Supervisor cancels the order.
11. If the vendor provides the tracking number within the processing time, the Procurement Supervisor collects the product once it is delivered.
12. At the same time, the Procurement Supervisor submits the payment to the vendor.
13. Once both steps are completed, the purchase process is considered finished, and the purchase is completed.
This process ensures that there is a clear and systematic approach to purchasing items, from the initial request to the final delivery and payment. Each step is important in maintaining accountability and efficiency in the procurement process.
Learn more about accountability here :-
https://brainly.com/question/29108212
#SPJ11
if co2 in the atmosphere increases 2ppm for every 10 gigatons of anthropogenic carbon that is added, what will the abundance of co2 be 50 years from now (assuming 'business as usual')?
Answers
It is estimated that atmospheric CO2 levels will continue to increase if significant actions are not taken to reduce greenhouse gas emissions.
According to the Global Carbon Project, in 2020, global carbon emissions were about 41 gigatons. If we assume this rate continues, then in 50 years, the emissions will be about 2050 gigatons, which would lead to an increase in CO2 in the atmosphere by about 100 ppm.
However, this estimate is based on current trends and does not take into account potential changes in emissions and other factors that could affect the rate of CO2 increase.
Learn more about Atmospheric CO2 here: https://brainly.com/question/14989493
#SPJ4
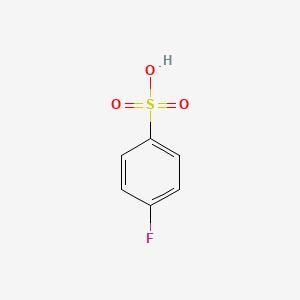
p‑fluoroanisole reacts with sulfur trioxide and sulfuric acid. draw the major product of this substitution reaction; if applicable, minimize formal charges via expanded octets. assume 1 equivalent of reagents is used.
Answers
The major product of the reaction between p‑fluoroanisole and sulfur trioxide and sulfuric acid is p‑fluorobenzene sulfonic acid. The sulfonic acid group (-SO3H) substitutes for the methoxy group (-OCH3) on the benzene ring. The product is shown below:
F
|
H3C--O--C6H4--SO3H
|
H
Note that the sulfur atom has an expanded octet, with 10 electrons in its valence shell.
One of the most well-known instances of an electrophilic aromatic substitution reaction is the reaction of p-fluoroanisole with sulphur trioxide and sulfuric acid. The electrophile in this reaction is a highly reactive sulphur trioxide-sulfuric acid complex, and the sulphur trioxide and sulfuric acid serve as its sources. The electrophile assaults the p-electron-rich fluoroanisole's aromatic ring, removing the methoxy group and replacing it with a sulfonic acid (-SO3H) group.
P-fluorobenzenesulfonic acid is a helpful intermediate in many chemical synthesis reactions due to the sulfonic acid group's high acidity and polarity. Additionally, a variety of chemical processes, including esterification, amidation, and reduction, can be used to further modify the sulfonic acid group in order to produce other derivatives.
The sulfonic acid group's sulphur atom has an extended octet, which implies it contains more than eight valence electrons in its outer shell. For lesser elements like carbon, nitrogen, and oxygen, this is unusual, but for heavier elements like sulphur and phosphorus, it is rather typical. Expanded octets are typically seen when the central atom can interact with empty d orbitals that can form bonds.
For more question on substitution reaction click on
https://brainly.com/question/27906533
#SPJ4
2. How many grams are in 7.46 x 10^22 molecules of nitrogen trihydride?
Answers
What would make oppositely charged objects attract each other more?
increasing the positive charge of the positively charged object and increasing the negative charge of the negatively
charged object
decreasing the positive charge of the positively charged object and decreasing the negative charge of the negatively
charged object
increasing the distance between the positively charged object and the negatively charged object
maintaining the distance between the positively charged object and the negatively charged object
Answers
Answer: increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged object.
Explanation:
edge
Answer:
Your answer is A.
Explanation:
Methanol, propan-1-ol and ethanol has boiling point of 65 degree celsius, 97 degree celsuis, 87 degree celsuis respectively. A student uses fractional distillation to separate a mixture of these compound. State which liquid will be collected in the second fraction and explain why
Answers
Answer:
ethanol
Explanation:
Methanol has a boiling point of 65°C, propan-1-ol has a boiling point of 97°C and ethanol has a boiling point of 87°C
Fractional distillation is used for separating miscible liquids through the use of the different vapor pressure properties of the liquids. As the mixture is heated up, components start to vaporize according to their boiling points. The component with the least boiling point vaporizes first while the component with the highest boiling point vaporizes last.
This means that methanol would vaporize at the first fraction because it has the lowest boiling point followed by ethanol at the second fraction and then propan-1-ol at the last fraction.
How dose critical mass play a role in nuclear reaction?
Answers
Answer:
How does critical mass play a role in a nuclear reaction?
Explanation:
It is the minimum amount of material needed to start a fission reaction. ... It is the minimum amount of material needed to sustain a fission reaction.
What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? 2P(s) + 3I2(s) -> 2PI3 (s) a. Which is the limiting reactant? Justify your answer. b. What is the theoretical yield of PI3? c. How much of the excess reagent will be left unused after the reaction?
Answers
Calculate the proportion of excess reagent eaten from the earth's weight of element or compound provided to determine the amount of surplus reactant that is still present.
After the reaction, what occurs to the extra reactant?A reversible reaction can be converted more often by using one of the reaction mixture in excess. Due to the Le Create a win - win principle, if too much reactant is applied, the reaction balance will change in favour of the products.
Which reagent is excess and which chemical is the least reactive?The limiting reagent is the substance that will completely disappear during a pharmacological reaction. If that reactant is used up, the reaction cannot go on. Hence, it stops the reaction from escalating. The additional reagent would also have kept reacting if the other reactivity hadn't been consumed.
To know more about compound visit:
https://brainly.com/question/13516179
#SPJ1
Charlie is frying an egg in a pan located over a gas burner. He develops a model to determine the energy produced by the flame in the gas
burner by calculating the energy absorbed by the egg. Which assumption will Charlie need to make in order for his model to be considered a
closed system?
A. Heat flows from the egg to the surroundings.
O
B. Heat flows from the pan to the surroundings.
O
C. No heat is lost from the flame or the egg to the surroundings.
OD. The egg receives heat from the flame and the surroundings.
Answers
A closed system is one in which there is no exchange of materials or energy with the surroundings hence the correct assumption is that "No heat is lost from the flame or the egg to the surroundings."
Another name for a closed system is an isolated system. In such system, there is exchange of material or energy between the system and the surroundings.
In this case, the system comprises of the pan and the eggs. If the system is to be a closed system then " No heat is lost from the flame or the egg to the surroundings."
Learn more; https://brainly.com/question/8987993
A _ serves as a long-term storage area for water or nutrients.
answer) reservoir
Answers
A cylindrical water tank is being filled at a rate of LaTeX: 10\:m^3\:10 m 3 per minute and is leaking at a rate of LaTeX: 3\:m^33 m 3 per minute. If the water level is changing at a rate of LaTeX: \frac{4}{\pi}4 π meters/minute when the height is at 6 meters. What is the radius of the tank?
Answers
Answer:
1.323 m
Explanation:
Rate of filling = \(10\ \text{m}^3/\text{min}\)
Rate of leakage = \(3\ \text{m}^3/\text{min}\)
Net change in volume of water = \(10-3=7\ \text{m}^3/\text{min}\)
\(\dfrac{dh}{dt}\) = Rate of change of height = \(\dfrac{4}{\pi}\ \text{m/min}\)
Volume of cylinder is given by
\(V=\pi r^2h\)
Differentiating with respect to time we get
\(\dfrac{dV}{dt}=\pi r^2\dfrac{dh}{dt}\\\Rightarrow 7=\pi r^2\times \dfrac{4}{\pi}\\\Rightarrow r=\sqrt{\dfrac{7}{4}}\\\Rightarrow r=1.323\ \text{m}\)
The radius of the tank is 1.323 m.
What type of quantity is momentum
Answers
Answer:
Vector quantityExplanation:
Momentum is a vector quantity because it has both magnitude and direction
Which statement best explains how the passage fits
the realistic fiction genre?
Answers
Answer:
It seems believable and familiar.
Explanation:
Plz mark Brainlest
what is the total number of valence electrons in the lewis structure of sef2o ? electrons draw a lewis structure for sef2o .
Answers
The total number of valence electrons in the Lewis structure of SeF2O is 26.
To determine the total number of valence electrons in the Lewis structure of SeF2O, we need to consider the valence electrons contributed by each atom.
Sulfur (S) is in Group 16 of the periodic table and has 6 valence electrons.
Fluorine (F) is in Group 17 of the periodic table and has 7 valence electrons.
Oxygen (O) is in Group 16 of the periodic table and also has 6 valence electrons.
For SeF2O, we have:
1 atom of S, which contributes 6 valence electrons
2 atoms of F, which contribute 2 × 7 = 14 valence electrons
1 atom of O, which contributes 6 valence electrons
Total valence electrons = 6 + 14 + 6 = 26
Therefore, the total number of valence electrons in the Lewis structure of SeF2O is 26.
To draw the Lewis structure of SeF2O, we can follow these steps:
1. Determine the central atom: In this case, Sulfur (S) is the central atom since it is less electronegative than Oxygen (O) and Fluorine (F).
2. Connect the outer atoms to the central atom using single bonds: Attach the two Fluorine atoms (F) to the Sulfur atom (S) using single bonds.
F-S-F
3. Assign remaining valence electrons to outer atoms: After connecting the Fluorine atoms (F) to the Sulfur atom (S), we have 26 - 2 = 24 valence electrons remaining.
4. Place remaining valence electrons on the central atom: The remaining 24 valence electrons will be placed on the central atom, Sulfur (S), and Oxygen (O), taking into account the octet rule.
F-S-F
|
O
5. Check octet rule and adjust if necessary: In this case, the Sulfur (S) atom has 8 electrons (full octet) and the Oxygen (O) atom has 6 electrons. Since Oxygen can accommodate more than 8 electrons, we can leave it with 6 electrons.
The final Lewis structure of SeF2O will have a single bond between Sulfur (S) and each Fluorine (F) atom, and a lone pair on the Oxygen (O) atom.
Learn more about Lewis structure at: https://brainly.com/question/13820123
#SPJ11
Mobile water supply operations, such as water shuttles or relay pumping, must be performed:_____.
Answers
Mobile water supply operations, such as water shuttles or relay pumping, must be performed In rural areas without public water distribution systems
A distribution systems includes all of the centers and devices connecting a transmission device to the customer's device. A traditional distribution device can consist of: Substations. Distribution Feeder Circuits. Switches.
Distribution systems, additionally referred to as dispensed computing, is a device with more than one additive placed on extraordinary machines that speak and coordinate movements so that it will seem like an unmarried coherent device to the end-user.
A dispensed device is computing surrounding wherein diverse additives unfold throughout more than one computer (or different computing gadgets) on a network. These gadgets cut up the work, coordinating their efforts to finish the task extra effectively than if an unmarried tool were accountable for the task.
Learn more about distribution systems here;
https://brainly.com/question/27905732
#SPJ4
Identify the following for an atom with 22 protons, 22 electrons, and a mass number of
48 amu.
a. Atomic number:
b. Number of neutrons:
c. Nuclear Notation:
d. Hyphen Notation:
please help
Answers
a. The atomic number is the number of protons in an atom. In this case, the atom has 22 protons, so the atomic number is 22.
b. The number of neutrons can be calculated by subtracting the atomic number (number of protons) from the mass number. In this case, the mass number is given as 48, and the atomic number is 22. Therefore, the number of neutrons is 48 - 22 = 26.
c. Nuclear notation represents the composition of an atom in a compact form. For the given atom with 22 protons and 26 neutrons, the nuclear notation is written as follows:
^48Ti (where the superscript represents the mass number and the subscript represents the atomic number)
d. Hyphen notation is another way to represent the composition of an atom. It includes the element symbol, followed by the mass number, separated by a hyphen. For the given atom, the hyphen notation is:
Titanium-48
Learn more about Titanium-48 here -: brainly.com/question/29793330
#SPJ11
ayudenme porfa doy corona xd

Answers
Answer:
e) 5
Explanation:
Because it's H
an evacuated cylinder has a volume of 50 liters. if 20 liters of nitrogen fas and 20 liters of oxygen gas are pumped into this evacuated cylinder, how much of the cylinder is filled with the two gasses?
Answers
The nitrogen gas and the oxygen gas will both occupy 50 liters.
Volume of a gasLet us recall form the kinetic theory of gases that a gas is in constant random motion. The gas does not have a specific volume but takes on the volume of the container since it will expand and fill the container.
The volume occupied by the two gases will be the same as the volume of the container. Hence, the nitrogen gas and the oxygen gas will both occupy 50 liters.
Learn more about volume of a gas: https://brainly.com/question/4519953
Answer:
B. The entire container because gases will expand to fill it.
Explanation:
I took to test, plus it directly says this in the lesson
what are two bones that make blood cells.
(name of the bones) :)
Answers
Answer:
Long bones contain yellow bone marrow and red bone marrow, which produce blood cells.
Explanation:
Have a nice night!
The majority of your blood cells are produced in your bone marrow. This is known as haemopoiesis. In children, haemopoiesis occurs in the long bones, such as the thighbone (femur). Adults have it mostly in their spine (vertebrae), hips, ribs, skull, and breastbone (sternum).
What is haemopoiesis ?Hematopoiesis is the process by which all the cellular components of blood and blood plasma are produced. It happens in the hematopoietic system, which includes organs and tissues like bone marrow, liver, and spleen. Simply put, hematopoiesis is the process by which the body produces blood cells.
The bone marrow is where blood cells are created. The soft, spongy material in the center of the bones is called bone marrow. It generates approximately 95% of the body's blood cells. The pelvic bones, breastbones, and spine bones contain the majority of the adult body's bone marrow.
Red cells are constantly produced in the marrow of certain bones. As previously stated, the marrow is the primary site of red cell production, known as erythropoiesis, in adults.
Thus, The majority of your blood cells are produced in your bone marrow. This is known as haemopoiesis.
To learn more about haemopoiesis, follow the link;
https://brainly.com/question/24372216
#SPJ2
(iii) If paper products accounted for 33% of the total MSW recycled in 2010 and about 7 m^3 of landfill
space is saved for each metric ton of paper products recycled, calculate how much landfill space was
saved by recycling paper products in 2010 in the United States.
Answers
The amount of landfill space saved by recycling paper products in 2010 in the United States is 2.31 times the total amount of MSW recycled in that year.
To calculate the amount of landfill space saved by recycling paper products in 2010 in the United States, we need to find out the number of paper products that were recycled. We can do that by using the data on the total MSW recycled and the percentage of that which was accounted for by paper products.
Percentage of total MSW recycled accounted for by paper products in 2010 = 33%
Amount of landfill space saved per metric ton of paper products recycled = 7 m³
We need to determine the amount of landfill space saved by recycling paper products in 2010 in the United States.
Therefore, Let's assume that the total amount of MSW recycled in 2010 was x metric tons. Then, the number of paper products recycled = 33% of x= (33/100) x metric tons = 0.33x metric tons
Therefore,
The amount of landfill space saved by recycling paper products in 2010 = Amount of paper products recycled × Landfill space saved per metric ton of paper products recycled= 0.33x metric tons × 7 m³/metric ton= 2.31x m³
You can learn more about landfills at: brainly.com/question/31946850
#SPJ11
The equilibrium concentrations were determined to be: NCI3 = 0.5 M, N2 = 0.18 M and C12 = 0.25 M. What is the Kc value for this reaction?
Answers
When a chemical process reaches equilibrium, the equilibrium constant (often represented by the letter K) sheds light on the interaction between the reactants and products. The value of Kc is 88.96.
The ratio of the concentration of the products to the concentration of the reactants, each raised to their respective stoichiometric coefficients, is the equilibrium constant of concentration (denoted by Kc) of a chemical process at equilibrium.
Here the reaction is:
N₂ + 3Cl₂ → 2NCl₃
Kc = [NCl₃]² / [N₂] [Cl₂]³
Kc = (0.5)² / (0.18) (0.25)³ = 88.96
To know more about equilibrium constant, visit;
https://brainly.com/question/29253884
#SPJ1
The atmospheric pressure on top of Mt. Everest (elevation 29,028) is 250. torr. Calculate the atmospheric pressure in mmHg and atm. Round each of your answers to 3 significant digits.
Answers
The atmospheric pressure on top of Mt. Everest is 250 mmHg and 0.328 atm, when rounded to 3 significant digits.
What is Pressure?Pressure is a force that is exerted over a surface area. It is the amount of force applied to an object per unit area. Pressure is typically expressed in units of force per unit of area, such as pounds per square inch (psi) or pascals (Pa). Pressure is an important factor in many areas of engineering, physics, chemistry, and biology.
Atmospheric pressure can be measured in torr (1 Torr = 1mmHg), atm (1 atm = 760mmHg) or in kPa (1 atm = 101.3kPa).
250 torr = 250 mmHg
250 mmHg / 760 mmHg = 0.328 atm
To learn more about Pressure
https://brainly.com/question/30235826
#SPJ1
Identify the number of atoms for each element in 8Sio2?
Answers
Explanation:
4.1 The Chemical Equation
LEARNING OBJECTIVES
Define chemical equation.
Identify the parts of a chemical equation.
A chemical reaction expresses a chemical change. For example, one chemical property of hydrogen is that it will react with oxygen to make water. We can write that as follows:
hydrogen reacts with oxygen to make water
We can represent this chemical change more succinctly as
hydrogen + oxygen → water
where the + sign means that the two substances interact chemically with each other and the → symbol implies that a chemical reaction takes place. But substances can also be represented by chemical formulas. Remembering that hydrogen and oxygen both exist as diatomic molecules, we can rewrite our chemical change as
H2 + O2 → H2O
This is an example of a chemical equation, which is a concise way of representing a chemical reaction. The initial substances are called reactants, and the final substances are called products.
Unfortunately, it is also an incomplete chemical equation. The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. If we count the number of hydrogen atoms in the reactants and products, we find two hydrogen atoms. But if we count the number of oxygen atoms in the reactants and products, we find that there are two oxygen atoms in the reactants but only one oxygen atom in the products.
What can we do? Can we change the subscripts in the formula for water so that it has two oxygen atoms in it? No; you cannot change the formulas of individual substances because the chemical formula for a given substance is characteristic of that substance. What you can do, however, is to change the number of molecules that react or are produced. We do this one element at a time, going from one side of the reaction to the other, changing the number of molecules of a substance until all elements have the same number of atoms on each side.
To accommodate the two oxygen atoms as reactants, let us assume that we have two water molecules as products:
H2 + O2 → 2H2O
The 2 in front of the formula for water is called a coefficient. Now there is the same number of oxygen atoms in the reactants as there are in the product. But in satisfying the need for the same number of oxygen atoms on both sides of the reaction, we have also changed the number of hydrogen atoms on the product side, so the number of hydrogen atoms is no longer equal. No problem—simply go back to the reactant side of the equation and add a coefficient in front of the H2. The coefficient that works is 2:
2H2 + O2 → 2H2O
There are now four hydrogen atoms in the reactants and also four atoms of hydrogen in the product. There are two oxygen atoms in the reactants and two atoms of oxygen in the product. The law of conservation of matter has been satisfied. When the reactants and products of a chemical equation have the same number of atoms of all elements present, we say that an equation is balanced. All proper chemical equations are balanced. If a substance does not have a coefficient written in front of it, it is assumed to be 1. Also, the convention is to use all whole numbers when balancing chemical equations. This sometimes makes us do a bit more “back and forth” work when balancing a chemical equation.