Answers
Answer:
q
Explanation:
Related Questions
What are the four main reasons listed for why we would want to clone things?
Answers
Answer:
1: to preserve 2: save a species 3: it's just cool
Please I have a D in chem and grades close tomorrow ;-;
Which of the following polyatomic lons will form an lonic compound with a single sodium lon?
co₂2-
нсо3^1-
S04^2-
S03^2-
Answers
is the maximum population that a given area can support.
Answers
Answer: carrying capacity
Explanation:
Answer:
A
Explanation:
Carrying Capacity
Gluconolactone is a(n) _________________________, classified as a(n) _______________, that cyclizes to produce a(n) ___________________. C-1 oxidized derivative of glucose Ester of gluconic acid Aldonic acid
Answers
Answer:
gravity
Explanation:
a vessel containing the following gases is analyzed by a scientist: he, ne, ar, kr which of the following gases would be moving the slowest? a. Ne b. Ar c. Kr d. He
Answers
The gas moving the slowest would be Argon (Ar). The speed of a gas molecule is determined by its temperature, which is related to its average kinetic energy.
The gas moving the slowest would be Argon (Ar). The speed of a gas molecule is determined by its temperature, which is related to its average kinetic energy. In general, the heavier a gas molecule is, the slower it will move, as it takes more energy to get it moving. Among the gases listed, Argon has the heaviest molecular weight and therefore would be expected to move the slowest. Helium (He) has the lightest molecular weight and would be expected to move the fastest, followed by Neon (Ne), Krypton (Kr), and Argon (Ar) in order of increasing molecular weight.
Learn more about gases here: brainly.com/question/14812509
#SPJ4
1.02g of Na2SO4(M=142.04gmol-1) is dissolved in water to prepare 0.5000dm cubed of solution. What is the concentration of Na2SO4 in mol dm-3

Answers
Answer:
\(1.000 \times {10}^{2} \)
What do you think all the stuff around you and in the universe itself is made from??
Answers
The universe contains practically incalculable numbers of planets, moons, asteroids, comets, and clouds of dust and gas, all of which are whirling around in the expanse of space together with billions of galaxies and trillions of stars.
What Is the Universe Made of?Helium is the second most prevalent element in the universe after hydrogen, and the two together make up almost all ordinary stuff. But only a little portion of the universe—about 5%—is represented by this. Everything else is constructed of materials that are invisible and can only be identified indirectly. About 100 seconds after the Big Bang, the temperature dropped to a still-seething 1 billion degrees Kelvin, during which all of the matter that makes up the known elements in the periodic table, as well as all other objects in the universe, such as black holes, massive stars, and specks of space dust, were created.About 380,000 years later, the universe had cooled to the point that protons and neutrons could combine to make lithium, helium, and the hydrogen isotope deuterium, while free electrons were trapped to create neutral atoms.To learn more about Big bang theory, refer
https://brainly.com/question/10865002
#SPJ10
H3PO4 + Ca(OH)2 ----> H2O + Ca3(PO4)2 If 10.3g of Ca(OH)2 reacts , How much water is made
Answers
Taking into account the reaction stoichiometry, if 10.3 g of Ca(OH)₂ reacts, 5.01 grams of H₂O are formed.
Reaction stoichiometryThe balanced reaction is:
2 H₃PO₄ + 3 Ca(OH)₂ → 6 H₂O + Ca₃(PO₄)₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₃PO₄: 2 molesCa(OH)₂: 3 molesH₂O: 6 molesCa₃(PO₄)₂: 1 moleThe molar mass of the compounds is:
H₃PO₄: 98 g/moleCa(OH)₂: 74 g/moleH₂O: 18 g/moleCa₃(PO₄)₂: 310 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₃PO₄: 2 moles ×98 g/mole= 196 gramsCa(OH)₂: 3 moles ×74 g/mole= 222 gramsH₂O: 6 moles ×18 g/mole= 108 gramsCa₃(PO₄)₂: 1 mole ×310 g/mole= 310 gramsMass of water formedThe following rule of three can be applied: if by reaction stoichiometry 222 grams of Ca(OH)₂ form 108 grams of H₂O, 10.3 grams of Ca(OH)₂ form how much mass of H₂O?
mass of H₂O= (10.3 grams of Ca(OH)₂ ×108 grams of H₂O)÷ 222 grams of Ca(OH)₂
mass of H₂O= 5.01 grams
Finally, 5.01 grams of H₂O are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
What mass of H2 forms when
35.25 g Al reacts with excess
hydrochloric acid?

Answers
The mass of \(H_2\) that will be formed when 35.25 g Al reacts with excess hydrochloric acid would be 3.93 g.
Stoichiometric problemThe equation of the reaction of aluminum metal with hydrochloric acid to produce hydrogen gas is represented below:
\(2Al + 6HCl -- > 2AlCl_3 + 3H_2\)
From the equation, one would see that the mole ratio of aluminum to the hydrogen gas produced is 2:3.
Now, with 35.25 g of aluminum, recall that: mole = mass/molar mass.
The molar weight of aluminum is 27 g/mol.
Thus:
Mole of 35.25 g Al = 35.25/27
= 1.31 mol
From the mole ratio, the mole of hydrogen that will be produced would be:
3/2 x 1.31 =1.97 mol
The molar mass of \(H_2\) is 2 g/mol, the mass of 1.97 mol hydrogen gas can be calculated as:
Mass = mole x molar mass
= 1.97 x 2
= 3.93 grams
In other words, the amount of \(H_2\) that would be formed when 35.25 g All reacts with excess hydrochloric acid would be 3.93 g.
More on stoichiometric problems can be found here: https://brainly.com/question/28297916
#SPJ1
the oven thatb hardnens clay products to a temperature and put them to an irreversible state to their original material (clay) and are tremed as ceramics is
Answers
A kiln is a specialized oven used in ceramic art to harden clay objects to a temperature that causes irreversible chemical changes in the clay, resulting in a permanent transformation of the material into ceramics.
What is a kiln and how is it used in ceramic art?
A kiln is a specialized oven used in ceramic art to harden clay objects to a temperature that causes irreversible chemical changes in the clay, resulting in a permanent transformation of the material into ceramics.
The temperature required for this transformation depends on the type of clay being used and the desired final product, but it typically ranges from around 1,000 to 1,400 degrees Celsius (1,800 to 2,550 degrees Fahrenheit). Kilns can be used for a variety of ceramic techniques, including firing, glazing, and decorating clay objects.
To learn more about chemical changes, visit: https://brainly.com/question/1222323
#SPJ1
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
6:53
Acellus
Binary Molecular Compounds
What is the formula of this
binary molecular
compound?
carbon tetrabromide
A. CBr
4
B. CaBr
2
C. 4BrC
D. CaBr
all?
4
6
48
Help Resources
Answers
The formula for carbon tetrabromide is CBr4.
What is molecular structure?Molecular structure refers to the three-dimensional arrangement of atoms in a molecule and the chemical bonds that hold those atoms together. The molecular structure of a compound determines its physical and chemical properties, including its reactivity, solubility, boiling point, and melting point. The arrangement of atoms in a molecule is determined by the number and types of atoms present, as well as the strength and directionality of the chemical bonds between them. The shape of a molecule is also influenced by the presence of lone pairs of electrons, which can affect the overall geometry of the molecule.
Here,
This compound is made up of one carbon atom and four bromine atoms. The prefix "tetra" indicates that there are four bromine atoms present in the molecule. Carbon tetrabromide is a colorless, nonflammable, and dense liquid that was once used as a fire extinguishing agent. However, due to its potential toxicity, it is no longer widely used for this purpose.
To know more about molecular structure,
https://brainly.com/question/503958
#SPJ1
Cassini has a mass of 2523 kg, and Saturn
has a mass of 5.68 x 1026 kg. Saturn's radius
is 54,364 km. If Cassini feels a gravitational
force of 2.980 x 104 N, how high above
Saturn's surface is it?
Rearrange F gravity Gm,m₂/r2
to solve this problem
In 10 words or fewer, how high above Saturn's surface is the Cassini
satellite?
Answers
The F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and height is 108,728 km.
What is gravity?Gravity is the amount of force that is produced by the earth to attract the object toward the surface and it doubles if the mass is double.
The height of Saturn is the duble of the radius of the given radius of 54,364 km of the planet Saturn which is 108,728 km.
Therefore, F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and hight is 108,728 km.
Learn more about gravity, here:
https://brainly.com/question/4783082
#SPJ1
How many atoms are in 2.5mol of sodium
Answers
Answer:
1024 sodium atoms.
CHALLENGE #5: Oxalic acid is a weak, diprotic acid. Write a balanced equation for the complete neutralization for oxalic acid with sodium hydroxide. Find the values for the Ka's of oxalic acid in your textbook. What do you think the titration curve for oxalic acid would look like?
Sketch a rough titration curve based on the above information.
Answers
To write a balanced equation for the complete neutralization of oxalic acid (H2C2O4) with sodium hydroxide (NaOH), we need to consider that oxalic acid is diprotic, meaning it can donate two protons (H+) per molecule.
The balanced equation is:
H2C2O4 + 2 NaOH → Na2C2O4 + 2 H2O
Oxalic acid has two dissociation constants, also known as acid dissociation constants (Ka), due to its two ionizable hydrogen atoms:
Ka1 = 5.9 × 10^-2
Ka2 = 5.1 × 10^-5
The titration curve for oxalic acid would have two equivalence points, corresponding to the neutralization of each hydrogen ion in oxalic acid. The first equivalence point would occur when half of the oxalic acid has been neutralized, meaning that one hydrogen ion has reacted with sodium hydroxide.
At this point, the pH would be equal to the pKa1 of oxalic acid, which is approximately 1.23. The second equivalence point would occur when all of the oxalic acid has been neutralized, meaning that both hydrogen ions have reacted with sodium hydroxide. At this point, the pH would be equal to the pKa2 of oxalic acid, which is approximately 4.38.
The titration curve will look somewhat like a typical titration curve for a weak acid but with two distinct equivalence points due to its diprotic nature.
pH
^
| Equivalence point 2 (pH = 4.38)
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
| /
|/ Equivalence point 1 (pH = 1.23)
|----------------------------->
Volume of NaOH added
The initial pH of the solution would be acidic due to the presence of oxalic acid. As NaOH is added, the pH would gradually increase until it reaches the first equivalence point, where it would sharply increase due to the complete neutralization of one hydrogen ion.
After passing the first equivalence point, the pH would increase more gradually until it reaches the second equivalence point, where it would sharply increase again due to the complete neutralization of the second hydrogen ion. After passing the second equivalence point, the pH would be basic due to the excess of NaOH in solution.
For more question on neutralization click on
https://brainly.com/question/23008798
#SPJ11
In a coffee-cup calorimeter, 50.0 mL of 0.100 M AgNO3 and 50.0 mL of 0.100 M HCl are mixed to yield the following reaction:
Ag+ (aq) + Cl- (aq) → AgCl (s)
a. The two solutions were initially at 22.6 °C and the final temperature of the mixture
is 23.4 °C. Calculate the magnitude of heat energy that is transferred in this experiment. Assume that each individual solution has a density of 1.0 g/mL and a specific heat of 4.18 J/g°C.
b. Using the balanced equation above and your answer to part (a), calculate the value
of the enthalpy change (AH) of the reaction in units of kJ/mol.
c. According to your answer in part (b), is the precipitation of silver chloride an
endothermic or exothermic process? Briefly explain your choice.
Answers
The heat of reaction is -334.4 J.
Heat of reaction of AgCl is -66.88KJ/mol.
Precipitation of silver chloride is an exothermic process
Calculation:
We can calculate the heat using Calorimeter.
Formula of calorimeter is,
q = c.m.ΔT
Where,
q= heat of reaction, c= specific heat capacity
Now, mass of solution= 100g
ΔT = T2 - T1
Initial temperature (T1) = 22.60°c
Final temperature (T2) = 23.40°c
ΔT = 23.40°c - 22.60°C = 0.80°c
Now put all the value in the formula of calorimeter
q= c.m.ΔT
q= (4.18J/°c.g) × 100g × 0.80°c
q= 334.4 J
Heat will be released as the solution will get warmer
So, Heat of the reaction = -334.4 J
Let's consider the reaction between AgN\(O_{3}\) and CaC\(l_{2}\)
2 AgN\(O_{3}\) (aq) + CaC\(l_{2}\)(aq) ⇄ 2AgCl + Ca(N\(O_{3}\)\()_{2}\)
From this equation we get,
2 moles of AgN\(O_{3}\) produce 2 moles of AgCl
Means, 1 mole AgN\(O_{3}\) gives 1 mole AgCl.
So, the mole of AgN\(O_{3}\) in solution,
AgN\(O_{3}\); n= molarity of AgN\(O_{3}\) × volume of AgN\(O_{3}\) in litres
= 0.100 mol/L × 0.0500 L
= 0.0050 mol
This is mole of AgN\(O_{3}\) in solution = moles of AgCl formed = 0.0050 mol
To get heat of reaction in moles,
q= \(\frac{-334.4J}{0.0050 mol}\)
q = -66880 J/mol
q= -66.88KJ/mol
The heat of reaction for the AgCl formed
= -66.88KJ/mol
Precipitation of silver chloride is an exothermic process since the heat is released in this case.
What is an exothermic reaction?
The part of a solution that transforms into a different state while creating the solution or the part that isn't overly abundant; the substance that dissolves in another material.
To know more about exothermic reaction, check out https://brainly.com/question/2924714
#SPJ9
WHAT IS P=MV
EXPLAIN IN BREIF
Answers
Answer:
Potential energy = mass(velocity)
Explanation:
It is the formula used to explain how to find the answer for potential energy
How many moles are in 123.0 grams of KClO4? (3 points)
0.2354 mol KClO4
0.6445 mol KClO4
0.7124 mol KClO4
0.8878 mol KClO4
Answers
Answer:
0.8878 mol KClO4
Explanation:
Molar mass of KClO4 = 39.0983 + 35.453 + ( 15.9994 x 4 ) = 39.0983 + 35.453 + 63.9976 = 138.5489g/mol
but,
number of moles = mass in grams / molar mass where mass in grams = 123g, molar mass = 138.5489g/mol
number of moles = 123g / 138.5489g/mol = 0.8878mol
Therefore the number of moles present in 123 g of \( KClO_4\) is 0.888 moles.
Given,
The weight of the \( KClO_4\) is 123. grams
molecular weight of \( KClO_4\) is \(39+35.5+16\times4=138.5 g\)
138.5 g of \(KclO_4\) is equal to one mole
Gram molecular wt. of a substance is equal to one mole
No. of moles = \(\frac{Wt}{GMW} \)
123 g of \(KClO_4\) is equal to \(\frac{123}{138.5}=0.888 moles \)
Learn More:https://brainly.com/question/2416206
if ethanol is replaced by acetic acid would you expect the same change?
Answers
Explanation:
no because they have different functional group
Use the oxidation number method to balance the following equations: a) Al(s) + H2SO4 (aq) Al2(SO4)3 (aq) + H2 (g)
Answers
By using oxidation number method , the balance chemical equation is given by :
2Al + 3H₂SO₄ ------> Al₂(SO₄)₃ + 3H₂
the give equation is :
Al + H₂SO₄ ------> Al₂(SO₄)₃ + H₂
1) write the oxidation number of each atom
reactant product
Al 0 +2
H +1 0
S +6 +6
0 -2 -2
2) Identify the atom which is oxidized and reduced and balance the for these.
Al is oxidized, from 0 to +3 and hydrogen is reduced from +1 to 0.now to balance Al and H, multiply 2 on the right side
2Al + H₂SO₄ ------> Al₂(SO₄)₃ + H₂
3) calculate the total increase in oxidation number in oxidation and same for reduction.
2Al⁰ to 2Al⁺³ ( so total increase is +6)
2H⁺¹ to 2H⁰ ( decrease in -2 )
4) now, make total increase in oxidation number = total decrease in oxidation number .for this multiply 3 to hydrogen both side to get decrease of -6.
2Al + 3H₂SO₄ ------> Al₂(SO₄)₃ + 3H₂
There fore, the balance chemical equation by oxidation number method is given as :
2Al + 3H₂SO₄ ------> Al₂(SO₄)₃ + 3H₂
To learn more about oxidation number method here
https://brainly.com/question/27929526
#SPJ1
Which element or ion listed below has the electron configuration 1s22s22p63s23p6? Group of answer choices Cl K+ Na+ none of these Ca+
Answers
The electron configuration of the K+ ion is 1s22s22p63s23p6.
What are the four different electron configuration types?One orbital can accommodate a maximum of two electrons and comes in four different shapes: s, p, d, and f. Because they contain various sublevels, the p, d, and f orbitals may each hold more electrons. According to what was said, each element's position on the periodic table determines how its electrons are configured.
The electron configuration for potassium is written in what format?Potassium (K) has a complete electron configuration of 1s22s22p63s23p64s1. [Ar]4s1 is an acronym for the electron configuration of argon (Ar), plus one additional electron in the 4s orbital. The 18 electrons in argon. For 19 electrons of potassium, one more electron configuration completes the picture.
To know more about electron configuration visit:-
https://brainly.com/question/29757010
#SPJ4
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
Pick the word which best completes the sentence.
The weather man told our class that the ____ flows from west to east.
a.
air mass
c.
clouds
b.
jet stream
d.
cold front
Please select the best answer from the choices provided
A
B
C
D
Answers
Answer:
c
c
c
c
c
c
c
c
c
c
c
c
c
c
c
c
cc
c
c
c
c
c
c
c
c
c
ccc
c
c
c
c
c
c
c
c
c
c
c
c
c
c
c
c
c
cc
c
c
c
AMOUNG US AND THE ANSWER IS C
Explanation:
Answer:
its c
Explanation:
Which term is used to describe the variety
of inheritable traits in a species?
Ecosystem diversity
Genetic diversity
Natural selection
Species diversity
Answers
Genetic diversity refers to genetic variability within species. Each individual species possesses genes which are the source of its own unique features: In human beings, for example, the huge variety of people's faces reflects each person's genetic individuality.
So, Ecosystem Diversity is a term that describes how different habitats of organisms are. For example, the fact that our planet has deserts, tundras but also estuaries is a sign of the diversity of its ecosystems. Species diversity refers to the number of different species that inhabit a specific ecosystem or the whole biosphere; the more, the better for species diversity. Genetic diversity is the term we are looking for; it means the variation in genes (usually in one species) that leads to different inheritable traits (in members of the same species). For example eye color is an inheritable trait that showcases genetic diversity since there are many genes that determine a different color such as brown, blue, green etc. (even though the environment plays a role too). Finally, natural selection is a theory about the survival of the fittest due to competition. It relates to inheritable traits and their diversity but it does not describe them.
A gas has a pressure of 2.70 atm at 50.0 °C. What is the pressure at standard temperature (0°C)?
Answers
Answer:
2.282 atm
P1V1/T1 = P2V2/T2
2.70atm / (50+273) = X/ 273
make x subject of formula
:. X = 2.28 atm
or 2.28 * 1.01 *10⁵ N/m²
you can support by rating brainly it's very much appreciated ✅✅
If you somehow managed to isolate a single atom of lithium, how many neutrons would it probably have in its nucleus? Explain.
Answers
Answer:
3 neutrons
Explanation:
Lithium is 3 in the periodic table of elements. This means it has 3 protons usually most elements will have the same number of protons and neutrons in their standard form which you can see in lithium's atomic weight of 6.941.
Within which of Linnaeus's groupings are organisms most closely related to one another?
A) species
B) genus
C) phylum
D) kingdom
Answers
Answer:
B)Explanation:
A genus is a group that includes a number of very closely related species; a species within a genus includes populations of organisms that can potentially interbreed.
A study of the Earth by The National Aeronautics and Space Administration (NASA) determined there were 8.80×10¹¹ tons of
carbon stored in living plants on the Earth's surface, at the time of the study. Calculate the mass of carbon stored in these plants
in grams.
Answers
The mass of carbon stored in the plants in grams is 7.98×10¹⁴ Kg
Data obtained from the questionFrom the question given above, the following data were obtained:
Carbon (in ton) = 8.80×10¹¹ tons Carbon (in Kg) =?How to convert 8.80×10¹¹ tons to kilograms (Kg)We can convert 8.80×10¹¹ tons to kilograms as illustrated below:
1 ton = 907.19 Kg
Therefore,
8.80×10¹¹ tons = (8.80×10¹¹ tons × 907.19 Kg) / 1 ton
8.80×10¹¹ tons = 7.98×10¹⁴ Kg
Thus, 8.80×10¹¹ tons is equivalent to 7.98×10¹⁴ Kg
Therefore, we can say that the mass of the carbon in the plants is 7.98×10¹⁴ Kg
Learn more about conversion:
https://brainly.com/question/12974609
#SPJ1
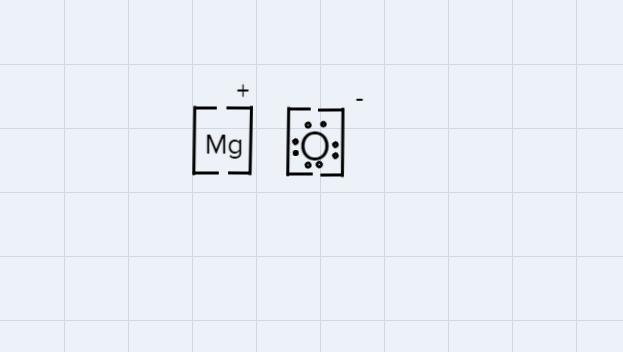
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?

Answers
Answer:
C.
Explanation:
The correct way to write the diagram of an ionic compound is:
- Use brackets by writing inside them, the symbol of each element separately.
- Draw the symbol and the number o the charge of each one, negative (-) add positive +()., in this case it is +1 for Mg (because Mg lost an electron) and -1 for O (because O won an elec).
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8