When hydrogen combines with ethylene (C2H4), ethane (C2H6) is formed. Write a balanced equation for this combination reaction.
Answers
The balanced chemical equation for the combination reaction between hydrogen and ethylene to form ethane can be represented as follows: C2H4 + H2 → C2H6.The chemical equation is already balanced.
The above chemical equation represents the combination reaction between hydrogen and ethylene to form ethane. The reaction is a simple example of a combination reaction. Combination reactions are reactions where two or more reactants combine to form a single product.
They are usually exothermic and release energy in the form of heat, light or sound. In the reaction above, ethylene and hydrogen combine to form ethane. Ethylene has two carbon atoms and four hydrogen atoms, while ethane has two carbon atoms and six hydrogen atoms. Therefore, hydrogen has been added to ethylene to form ethane.
To know more about balanced chemical equation refer here: https://brainly.com/question/28294176#
#SPJ11
Related Questions
naturally occuring element x exists in three isotopic forms: x-28 (27.343 amu, 67.14% abundance), x-29 (28.889 amu, 10.50% abundance), and x-32 (31.993 amu, 22.36% abundance). calculate the average atomic weight of x. please enter your answer to 4 significant figures.
Answers
The naturally occurring the element x exists in three isotopic forms. The average atomic weight of x 28.544 amu.
Given that :
x- 28, Abundance % = 67.14 % = 0.6714
The atomic mass = 27.343 amu
x-29, Abundance % = 10.50 % = 0.1050
The atomic mass = 28.889 amu
x- 32, Abundance % = 22.36 % = 0.2236
The atomic mass = 31.993 amu
The average atomic weight = ( 27.343 × 0.6714 ) + ( 28.889 × 0.1050) +
( 31.993 × 0.2236)
= 18.358 + 3.0333 + 7.153
= 28.544 amu
Thus, the average atomic weight of naturally occurring element x exists in three isotopic forms 28.544 amu.
To learn more about average atomic weight here
https://brainly.com/question/28013988
#SPJ4
I need help with Molar Ratio
______SO2(g) +_____O2(g) ------> _____SO3(g)
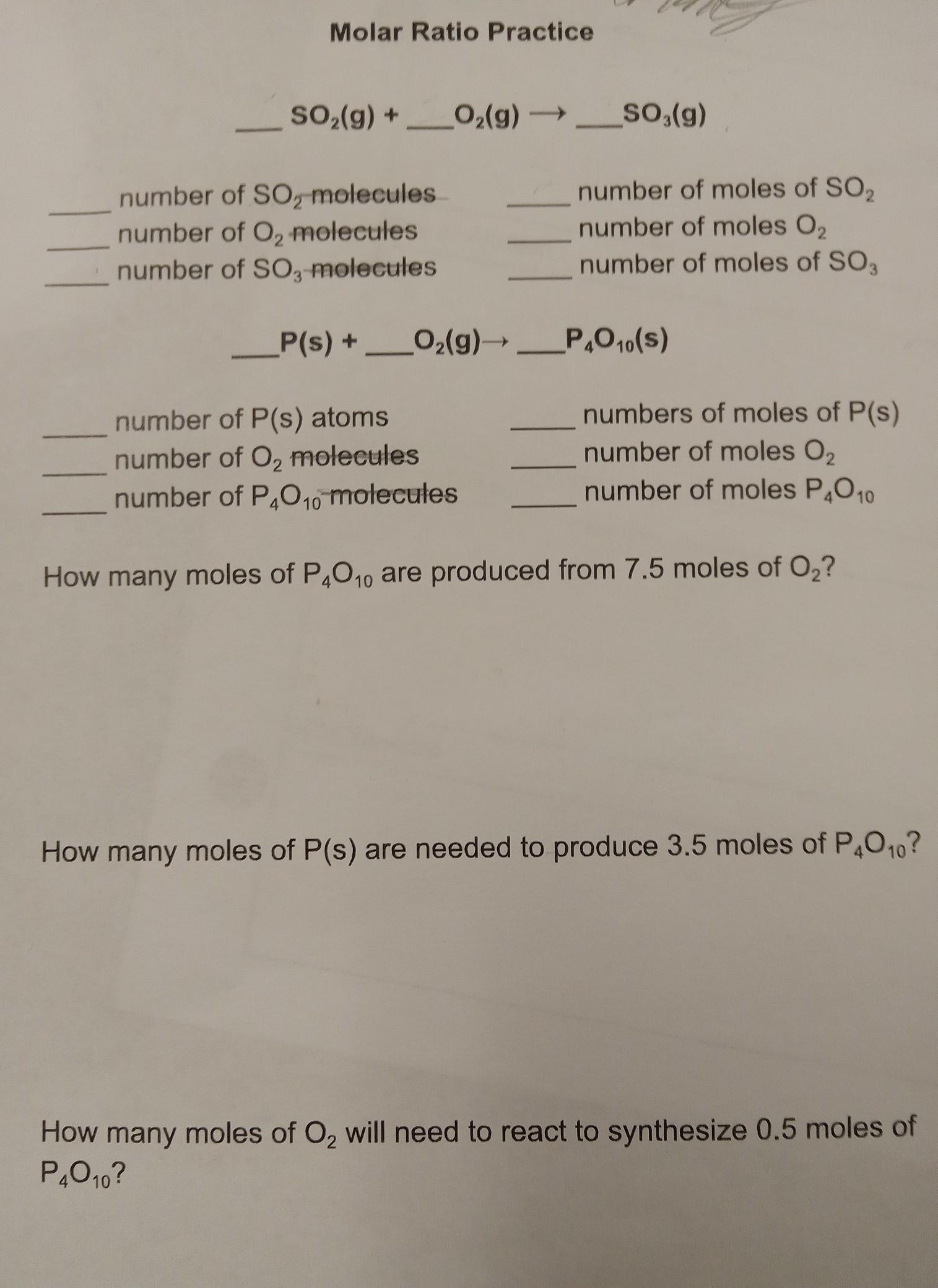
Answers
2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
1.204 * 10²⁴ number of SO₂ molecules = 2 number of moles of SO₂
6.02 * 10²³ number of O₂ molecules = 1 number of moles O₂
1.204 * 10²⁴ number of SO₃ molecules = 2 number of moles of SO,
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
2.408 * 10²⁴ number of P(s) atoms = 4 numbers of moles of P(s)
3.01 * 10²⁴ number of O₂ molecules = 5 number of moles O₂
6.02 * 10²³ number of moles P₄O₁₀ = number of P₄O₁₀ molecules
1.5 moles of P₄O₁₀ are produced from 7.5 moles of O₂.
14 moles of P(s) are needed to produce 3.5 moles of P₄O₁₀.
2.5 moles of O₂ will need to react to synthesize 0.5 moles of P₄O₁₀.
What is the mole ratio of the given reactions?The mole ratio of the given reactions is obtained from their equations of reaction.
1. 2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
The mole ratio is 2 : 1 : 2
1 mole of atoms or molecules contains 6.02 * 10²³ particles.
Hence, the number of particles is obtained by multiplying the number of moles by 6.02 * 10²³.
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
7.5 moles of O₂ will produce 7.5/5 moles of P₄O₁₀ = 1.5 moles of P₄O₁₀
3.5 moles of P₄O₁₀ will be produced by 3.5 * 4 moles of + = 14 moles of P(s)
0.5 moles of P₄O₁₀ will be produced by 0.5 * 5 moles of O₂ = 2.5 moles of O₂
Learn more about mole ratio at: https://brainly.com/question/30632038
#SPJ1
Calculate the freezing point of a nonionizing antifreeze solution containing 388 g ethylene glycol, C2H6O2, and 510 g of water.
Answers
Answer:
T° freezing solution = -22.8°C
Explanation:
To solve this problem we apply, the Freezing Point Depression. This is a colligative property which its formula is:
T° freezing pure solvent - T° freezing solution = Kf . molality . i
i = Van't Hoff factor.
We have been informed is a nonionizing solute, so i = 1
Our solute is ethylene glycol, so le'ts determine the moles to get molality
388 g . 1 mol / 62.07 g = 6.25 moles
molality (m) = moles of solute /kg of solvent
We convert mass of solvent, water, to kg → 510 g . 1kg /1000g = 0.510 kg
6.25 mol /0.510kg = 12.25 m
We replace at formula → 0°C - T° freezing solution = 1.86°C/m . 12.25 m . 1
T° freezing solution = -22.8°C
96. Phosphorus (P4) is commercially prepared by heating a mixture of calcium phosphate (Ca3(PO4)2), sand (SiO₂), and coke (C) in an electric furnace. The process involves
two reactions.
2Ca3(PO4)2(s) + 6SiO₂ (s)→ 6CaSiO3 (1) + P4O10(g)
P4010(g) +10C(s) → P4(g) + 10CO(g)
The P40 10 produced in the first reaction reacts with an excess of coke (C) in the second reaction. Determine the theoretical yield of P4 if 250.0 g of Ca3(PO4)2 and 400.0 g of SiO₂ are heated. If the actual yield of P4 is 45.0 g,
determine the percent yield of P4.
Answers
Percent yield is defined as the 100 times the ratio of actual yield and theoretical yield. It ca be calculated by finding theoretical yield of the reaction and come as 76.6%.
2Ca₃(PO₄)₂(s) + 6SiO₂(s) → 6CaSiO₃(l) + P₄O₁₀(g)
P₄O₁₀(g) + 10C(s) → P₄(g) + 10CO(g)
We need to find out the moles of Ca₃(PO₄)₂ and SiO₂ to find limiting reactant. With the help of limiting reactant we can find moles of P₄O₁₀ = Moles of P₄. We must convert moles of P₄ to mass using Molar mass (P₄ = 123.895g/mol):
Moles Ca₃(PO₄)₂ -Molar mass: 310.1767g/mol-
293.5g (1mol / 310.1767g) = 0.9462moles
Moles SiO₂ -Molar mass: 60.08g/mol-:
378.5g (1mol / 60.08g) = 6.30 moles
For the complete reaction of 6.30 moles of SiO₂ there are required:
6.30 moles SiO₂ (2 moles Ca₃(PO₄)₂ / 6 moles SiO₂) =
2.10 moles Ca₃(PO₄)₂. Now there are just 0.9462 moles, Ca₃(PO₄)₂ is limiting reactant
Moles of P₄O₁₀ = Moles P₄:
0.9462moles of Ca₃(PO₄)₂ (1mol P₄O₁₀ / 2 mol Ca₃(PO₄)₂)
= 0.4731 moles of P₄O₁₀ = Moles P₄.
The mass is calculated as:
0.4731 moles P₄ (123.895g / 1mol) = 58.6g = Theoretical yield.
Thus, Percent yield is calculated as:
44.9g / 58.6g ×100 = 76.6%
So, 76.6% is percent yield of P₄
To know more about percentage yield, please refer:
https://brainly.com/question/14531883
#SPJ9
What does it mean
"1. A chemical reaction. represents what happens in a chemical"
Answers
Eutrophication causes loss of Biodiversity”. Substantiate the statement
Answers
Eutrophication causes loss of biodiversity because many species need light to survive. It affects light penetration in the water body.
What is eutrophication?Eutrophication is a phenomenon where an aquatic environment (e.g., a lake) is enriched with minerals and nutrients.
The most common nutrients associated with eutrophication are nitrogen and phosphorus.
Eutrophication can severely affect light penetration in the water body, thereby affecting the survival of producer organisms (plants) and causing a loss of biodiversity across all trophic levels.
Learn more about Eutrophication here:
https://brainly.com/question/8499582
(1) how does dehydration reaction result in alkene synthesis?
Answers
Answer:
by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
Explanation:
Alcohols undergo E1 or E2 mechanisms to lose water and form a double bond. ... Therefore, the dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
Alcohols travel through either the E1 or E2 process to dry out and create a double bond. Therefore, in below given ways dehydration reaction result in alkene synthesis.
What is dehydration reaction?Condensation reactions include dehydration reactions. A water molecule is taken out of one of the reactants when the two chemicals combine, creating an unsaturated compound. The fact that water is always one of the results of a dehydration event is another clear indicators.
As a result, the dehydration process of alcohols to produce alkene is carried out by heating the alcohols at high temperatures in the presence of a powerful acid, such as sulfuric or phosphoric acid.
Therefore, in above given ways dehydration reaction result in alkene synthesis.
To know more about dehydration reaction, here:
https://brainly.com/question/29262706
#SPJ5
What does an atom's period tell us?
Answers
Answer:
A horizontal row in the periodic table, which signifies the totoal number of electron shells in an element's atom.
Explanation:
Hope this helpss)):
How many valence electrons does magnesium(Mg) have?
Answers
Magnesium have two valence electrons. Because the outer energy level for the magnesium atom is 3 and it has two electron in this energy level.
The Valence electrons are defined as the electrons that located in the outermost electron shell of an atom. These valence electrons being the furthest from the nucleus and thus the least tightly held by the atom are the electrons that participate in bonds and reactions. The number of valence electrons that an element has determines its reactivity, electronegativity and the number of bonds it can form. We can use the periodic table to help to determine how many valence electrons an element specifically a neutral atom of the element has. Looking at the group that the element is in as the group number indicates the number of valence electrons that the element has.
To learn more about Valence electron please visit:
https://brainly.com/question/371590
#SPJ4
Is very reactive and can readily combine with molecules containing atoms with lone pairs?
Answers
Fluorine is very reactive and can readily combine with molecules containing atoms with lone pairs.
Fluorine is a highly electronegative element, meaning it has a strong attraction for electrons. This makes it highly reactive as it seeks to gain electrons to achieve a stable electron configuration.
Molecules containing atoms with lone pairs, such as oxygen or nitrogen, are attractive to fluorine because they have unshared pairs of electrons that can be easily shared with fluorine. Fluorine can form strong bonds with these atoms, resulting in the formation of stable compounds.
In summary, fluorine is a highly reactive element that readily combines with molecules containing atoms with lone pairs. It forms strong bonds with these atoms, resulting in the formation of stable compounds.
Learn more about Fluorine -
brainly.com/question/30459929
#SPJ11
Match the vocabulary term with the correct definition.
hypothesis
[ Choose ]
observation
[ Choose ]
scientific inquiry
[ Choose ]
interpret
[ Choose ]
assume
[ Choose ]
![Match the vocabulary term with the correct definition. hypothesis[ Choose ]observation[ Choose ]scientific](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/aFsFLpBFJ12OE2yiFMa7F2nKeHMocG3m.png)
Answers
Answer:
I think the answer is assume
The Dutch scientist Johannes van der Waals developed a useful equation to predict the behavior of real gases. In the van der Waals equation, what are the constants a and b, respectively?
a. a is a measure of how strongly the gas molecules attract one another, and b is a measure of the finite volume occupied by the molecules.
b. a is a measure of the random motion of gas molecules, and b is a measure of the volume of the container.
c. a is a measure of the finite volume occupied by the molecules, and b is a measure of how strongly the gas molecules attract one another.
d. a is a measure of the molecular mass of the gas molecules, and b is a measure of the finite volume occupied by the molecules.
Answers
Answer:
Explanation:
The higher the value of a, the greater the attraction between molecules and the more easily the gas will compress.
The b term represents the excluded volume of the gas or the volume occupied by the gas particles.
1 mole Fe2(CrO4)3 = ____g Fe2 (CrO4)3.
Answers
Answer:
459.6711 grams
Explanation:
The awnser
How many significant figures are in $10,000,210
7
3
Answers
Answer:
7
Explanation:
Answer:
7
Explanation:
The significant figures are: 1,0,0,0,0,2,1
Remember the rules is
1. Non-digits are always significant.
2. Any zeros between two significant digits are significant.
3. A final zero or trailing zeros in the decimal portion only are significant
Those are three rules you have to follow in order to see how many significant figures there are in 10,000,210.
So I’m guessing 7 is the answer
Therefore the answer is 7.
Hope this helps! :)
Brainliest would be highly appreciated! Thanks! :)
What mass of reactants are used
during the reaction?
Iron + Sulfur➜ Iron Sulfide
27.50 g
? g
Give your answer to the correct number of
significant figures.
(g) Iron + Sulfur

Answers
Answer:
27.50 g
Explanation:
Unless weight was somehow gained/lost during the reaction, the products should weigh as much as the reactants. Since this isn't specified the mass of the reactants should be equal to the mass of the products.
Vinegar, which contains acetic acid, is used in foods and has few safety concerns. Hydrochloric acid is used in chemistry labs and requires the use of safety goggles and gloves. Why do the safety concerns for these two acids differ? 2 ... Acetic acid is a weak acid, and hydrochloric acid is a strong acid.
Answers
Answer:
acid
Explanation:
Long-term financial goals
O can keep you from furthering your education
O include spending money as you receive it
O mean buying what you want when you want it
sometimes conflict with short-term spending
Answers
Long-term financial goals involve strategic planning, saving, and investing to achieve financial security and major milestones. They may require making trade-offs with short-term spending, but they are not intended to hinder further education. Option D) is correct
Long-term financial goals refer to financial objectives that are set to be achieved over an extended period, typically spanning several years or even decades. These goals are focused on building wealth, financial security, and achieving specific milestones. They often involve saving, investing, and planning for major life events such as retirement, buying a home, or funding education.
It is important to note that long-term financial goals can sometimes conflict with short-term spending. This means that in order to achieve long-term financial goals, individuals may need to prioritize saving and investing over immediate gratification and discretionary spending. This might involve making sacrifices in the present, such as forgoing certain luxuries or reducing discretionary expenses, in order to allocate more resources towards long-term goals.
Long-term financial goals should not hinder further education. On the contrary, they can provide the necessary financial stability and resources to pursue higher education or professional development opportunities. They prioritize the allocation of funds towards education and skill-building, recognizing the long-term benefits and potential for higher income and career advancement.
While long-term financial goals may involve spending money as it is received, they do so with a purpose and strategic planning. They aim to allocate resources wisely, ensuring that spending is aligned with priorities and values, and that funds are also being allocated towards savings and investments to support long-term goals.
In summary, long-term financial goals involve strategic planning, saving, and investing to achieve financial security and major milestones. They may require making trade-offs with short-term spending, but they are not intended to hinder further education. They prioritize responsible financial management and align spending with long-term priorities. Therefore Option D) is correct.
For more question on investing
https://brainly.com/question/29359321
#SPJ8
Calcium is element 20 in the Periodic Table, has a mass of 40 amu and forms a 2+ ionic species. The calcium ion therefore has a. 18 protons, 18 neutrons and 22 electrons b. 22 protons, 18 neutrons and 18 electrons c. 20 protons, 20 neutrons and 18 electrons d. 18 protons, 20 neutrons and 20 electrons e. 20 protons, 18 neutrons and 20 electrons 1. In the following expression a∼1/b, what is the relationship between the components a and b ? a. Direct proportion b. None of the above c. Exact equation d. Inverse proportion e. Proportionality constant
Answers
The calcium ion has 18 protons, 20 neutrons, and 20 electrons.
The relationship between the components a and b is Inverse proportion.
The calcium ion (Ca2+) has a 2+ charge, indicating that it has lost 2 electrons from its neutral state. To determine the number of protons, neutrons, and electrons in the calcium ion, we need to consider its atomic number and mass.
The atomic number of calcium is 20, which indicates that it has 20 protons. Since the calcium ion has a 2+ charge, it means it has lost 2 electrons. Therefore, the number of electrons in the calcium ion is 20 - 2 = 18.
The mass number of calcium is 40 amu, which represents the total number of protons and neutrons. Since the calcium ion has 20 protons, the number of neutrons can be calculated as 40 - 20 = 20.
So, the correct option is: d. 18 protons, 20 neutrons, and 20 electrons
In the expression a∼1/b, the relationship between the components a and b is an inverse proportion. This means that as the value of a increases, the value of b decreases, and vice versa. The symbol ∼ represents the proportional relationship between a and 1/b, indicating that they are inversely related. Therefore, the correct answer is: Inverse proportion
To know more about calcium , click here, https://brainly.com/question/32135261
#SPJ11
4. The periodic table is organized into groups and periods of elements. The point
characteristics of a certain group of elements are listed below. Which of
these elements is in this group?*
Characteristics of a Group of Elements
• Is shiny
• Is solid at room temperature
• Has atoms with two valence electrons
O A Lithium
O B. Strontium
O C. Aluminum
O D. Silicon
Answers
Answer:B
Explanation:
Help me pretty please

Answers
Answer:
C
Explanation:
Answer:
I think ur answer would be C.)
Explanation:
hope this helps
choose atomic radius from the drop-down menu to see the relative sizes of the elements. in which parts of the table do you find the largest and smallest atoms?
Answers
The atoms of cesium (Cs) have the largest atomic radius on the periodic table.
Cesium has the chemical symbol Cs and the atomic number 55. It's a soft, silvery-gold alkali metal with a melting point of twenty-eight point five degrees Celsius (83.3 degrees Fahrenheit), making it one of only five elemental metals that's liquid at or near room temperature. Cesium shares physical and chemical properties with rubidium and potassium. Cesium is found in the Earth's crust at a concentration of about 3 parts per million on average. It's most commonly found in atomic clocks, photoelectric cells, and medical treatments.
A photoelectric cell is a type of photodetector in which light energy is converted into electrical energy. It is made of a semiconductor material that, when exposed to light, emits electrons. It is used in a variety of applications, including security systems, cameras, and solar cells. Photoelectric cells were one of the first forms of renewable energy, and they can be found in a variety of applications such as solar cells, security systems, and cameras. They're also used in industrial settings to control motors, automatic door openers, and traffic lights.
To know more about Cesium:
brainly.com/question/24316293
#SPJ4
what kind of bond is formed between the two hydrogen atoms and the single oxygen atom?
Answers
2. Aqueous phosphoric acid and aqueous calcium hydroxide reaction
form solid calcium phosphate and water. Write a balanced chemical
equation for this reaction.
Answers
Answer:
\(3Ca(OH)_{2} + 2H_3PO_4 ==> Ca_3(PO_4)_2 + 6H_2O\)
Explanation:
The chemical equation represented as:
\(2H_3PO_4 (aq)+ 3Ca (OH)_2 (aq) ----- > Ca(PO_4)_2 (s)+ 6H_2O (l)\)
What is Chemical equation?A chemical equation is defined as a symbolic representation of a chemical reaction in the form of symbols and chemical formulas. Coefficients that are used in front of reactants and products to balance the equation to show that matter is conserved.
Representation of chemical reactions by symbols and formulas of substances is called chemical equation. In this equation A and B are called reactants and C and D are called products while the arrow shows the direction of the chemical reaction.
For the above given information,
\(2H_3PO_4 (aq)+ 3Ca (OH)_2 (aq) ----- > Ca(PO_4)_2 (s)+ 6H_2O (l)\)
Phosphoric Calcium Calcium Water
acid hydroxide phosphate
Where, Aqueous phosphoric acid and aqueous calcium hydroxide are reactants and solid calcium phosphate and water are products.
Thus, the chemical equation represented as:
\(2H_3PO_4 (aq)+ 3Ca (OH)_2 (aq) ----- > Ca(PO_4)_2 (s)+ 6H_2O (l)\)
Learn more about Chemical equation, here:
https://brainly.com/question/28294176
#SPJ2
__Pb(NO3)2 + __NaCI = __NaNO3 + __PbCI2
Answers
Answer:
Pb(NO3)2 + 2NaCl = 2NaNO3 + PbCl2
What do all cells requiere a constant supply of
Answers
Gloria is making a model of an atom. She uses three different colors to represent the three basic particles that make up the atom. Which particles should she display in the nucleus of the atom?
Group of answer choices
electrons and protons
protons and neutrons
electrons only
neutrons only
Answers
Answer:
protons and neutrons
Explanation:
The nucleus of the atom contains protons and neutrons. The electrons of the atom orbit the nucleus.
Select the oxidation number for the element referenced in each example
below.

Answers
A positive or negative number assigned to an atom to reflect its degree of oxidation or reduction is known as an oxidation number.
Simply said, the number assigned to each element in the chemical mixture is what is known as the oxidation number. The number of electrons which atoms within a molecule may exchange, lose, or gain when forming chemical connections with other atoms from a different element is known as the oxidation number. The oxidation state is another name for the oxidation number.
To know more about oxidation number, here:
https://brainly.com/question/29100691
#SPJ1
The standard emf for the cell using the overall cell reaction below is +2.20 V:
2 Al(s) + 3 I2(s) → 2 Al3+(aq) + 6 I-(aq)
The emf generated by the cell when [Al3+] = 4.5 × 10-3 M and [I-] = 0.015 M is __________ V.
The standard emf for the cell using the overall cell reaction below is +2.20 V:
2 Al(s) + 3 I2(s) → 2 Al3+(aq) + 6 I-(aq)
The emf generated by the cell when [Al3+] = 4.5 × 10-3 M and [I-] = 0.015 M is __________ V.
2.05
2.35
2.21
2.51
2.24
Answers
The emf generated by the cell when [Al³⁺] = 4.5 × 10-3 M and [I⁻] = 0.015 M is 2.24 V.
Given the chemical equation,
2 Al(s) + 3 I₂(s) → 2 Al³⁺(aq) + 6 I⁻(aq)
The standard emf for the cell is given as +2.20 V.
To calculate the emf generated by the cell when [Al³⁺] = 4.5 × 10⁻³ M and [I⁻] = 0.015 M, we need to use the Nernst equation.
Nernst equation relates the measured or cell potential of a redox reaction to the standard electrode potential, temperature, reaction quotient, and the number of electrons transferred in the balanced chemical equation.
The Nernst equation can be represented as, Ecell = E°cell - (0.0592/n) logQ
Where,
E°cell is the standard electrode potential
n is the number of electrons transferred
Q is the reaction quotient
Ecell is the cell potential
Plugging the values in the equation,
Ecell = E°cell - (0.0592/n) logQ = +2.20 - (0.0592/6) log[(4.5 × 10-3)/(0.015)6/3] = +2.24 V
Therefore, the emf generated by the cell when [Al³⁺] = 4.5 × 10-3 M and [I⁻] = 0.015 M is 2.24 V.
Hence, the correct option is 2.24.
Learn more about emf at https://brainly.com/question/30083242
#SPJ11
c’est quoi un isotope
Answers
Answer:
c'eop wjau ciouwh
Explanation:
You were given a 100. G wine sample to verify its age. Using tritium dating you observe that the sample has 0. 688 decay events per minute. Tritium has a half life of 12. 3 and fresh water exhibits 5. 5 decay events per minute per 100g. What year was the wine produced?.
Answers
Wine was produced 37 years ago (1984 as usual year 15,2021) that is shown in the calculations below.
Reaction rate is calculated using the formula rate = Δ[C]/Δt, where Δ[C] is the change in product concentration during time period Δt. The rate of reaction can be observed by watching the disappearance of a reactant or the appearance of a product over time.
The time can be represented as follows:
t= 2.303\∧ log A0/A
∧= 0.693/t 1/2
The rate of a reaction is proportional to the reciprocal of the time taken. Rate α 1 time Rate is inversely proportional to time. Units: s-1, min-1 etc.
The given parameters are as follows:
t1/2=12.3
A0=5.5
A=0.688
t= 2.303/(0.693/12.3) log (5.5/0.688)
t=36.9
t=37 years
Thus, wine was produced 37 years ago (1984 as usual year 15,2021)
To learn more about rate of reaction check the link below:
https://brainly.com/question/24795637
#SPJ4