when 0.750 mol n2h4 is mixed with .500 mol h202, how much n2, in moles, is formed? be sure to use the limiting reagent.
Answers
When 0.750 mol of N2H4 is mixed with 0.500 mol of H2O2, the limiting reagent is H2O2, and the amount of N2 formed is 0.500 mol.
To determine the amount of N2 formed when 0.750 mol N2H4 reacts with 0.500 mol H2O2, we need to identify the limiting reagent.
Let's write the balanced chemical equation for the reaction:
N2H4 + H2O2 → N2 + 2H2O
According to the balanced equation, the stoichiometric ratio between N2H4 and N2 is 1:1. This means that for every 1 mole of N2H4 reacted, 1 mole of N2 is formed.
To find the limiting reagent, we compare the number of moles of each reactant to their respective stoichiometric coefficients in the balanced equation.
For N2H4: 0.750 mol
For H2O2: 0.500 mol
The stoichiometric coefficient of N2H4 is already 1, so no conversion is necessary. However, we need to convert the moles of H2O2 to moles of N2 using the stoichiometric ratio.
1 mol N2H4 : 1 mol N2
0.500 mol H2O2 : x mol N2
By applying the ratio, we find:
x = 0.500 mol N2
Now we compare the amounts of N2 produced from both reactants. Since the stoichiometric ratio indicates that 1 mole of N2H4 produces 1 mole of N2, and the stoichiometry of the limiting reagent is H2O2, we can conclude that only 0.500 mol of N2 will be formed.
Therefore, when 0.750 mol of N2H4 is mixed with 0.500 mol of H2O2, the limiting reagent is H2O2, and the amount of N2 formed is 0.500 mol.
learn more about N2H4 here
https://brainly.com/question/22638392
#SPJ11
Related Questions
What is the standard model? What can it explain?
Answers
Answer:
The Standard Model of particle physics is the theory describing three of the four known fundamental forces (the electromagnetic, weak, and strong interactions, and not including the gravitational force) in the universe, as well as classifying all known elementary particles. It was developed in stages throughout the latter half of the 20th century, through the work of many scientists around the world, with the current formulation being finalized in the mid-1970s upon experimental confirmation of the existence of quarks.
Answer:
practice physical
Explanation:
The Standard Model of particle physics is the theory describing three of the four known fundamental forces in the universe, as well as classifying all known elementary particles.
In which state is oxygen at -200°c
Answers
Answer:
At very low temperatures (about -183 degrees centigrade, oxygen becomes a pale blue liquid and even becomes a solid at about -218 degrees centigrade.
Explanation:
When a 16.50 g sample containing nickel and oxygen is analyzed, 5.87 g of nickel are found. What is the percent composition of this mineral?
Answers
Answer:
\(\%O=64.2\%\\\\\%Ni=35.8\%\)
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to tell that since the total mass is 16.50 g and 5.87 g correspond to nickel, then the mass of oxygen is:
\(m_O=16.50g-5.87g=10.63g\)
And therefore, the resulting percent composition turns out to be:
\(\%O=\frac{10.60}{16.50} *100\%=64.2\%\\\\\%Ni=\frac{5.87}{16.50} *100\%=35.8\%\)
Regards!
Percentage composition is the percentage of the mass of the individual element. The percentage composition of oxygen is 64.2% and of nickel is 35.8%.
What is mass?Mass is the amount of weight occupied by the sample or an element in a compound or molecule.
Given,
The total mass of the compound = 16.50 gm
Mass of nickel = 5.87 gm
The mass of oxygen is calculated as: 16.50 - 5.87 = 10.63 gm.
The percentage composition of oxygen is calculated as:
\(\dfrac{10.60}{16.50} \times 100\% = 64.2\%\)
The percentage composition of nickel is calculated as:
\(\dfrac{5.87}{16.50} \times 100\% = 35.8\%\)
Therefore, the percentage of oxygen is 64.25% and of nickel is 35.8%.
Learn more about mass percentage here:
https://brainly.com/question/9248060
Fluorine gas reacts with aqueous iron (II) iodine to produce iron (II) fluoride and iodine liquid What is the balanced chemical equation for this reaction?
Answers
Answer:
F₂ (g) + FeI₂ (aq) → FeF₂ (aq) + I₂ (l)
Explanation:
Our reactants are:
F₂ → Fluorine gas, a dyatomic molecule
FeI₂ → Iron (II) iodine
Our products are:
I₂ → Iodine
FeF₂ → Iron (II) fluoride
Then, the reaction is:
F₂ (g) + FeI₂ (aq) → FeF₂ (aq) + I₂ (l)
We see it is completely balanced.
Find the H+ concentration of of a solution that has a pH solution of 12.24
Answers
Answer:
[H⁺] = 5.754 x 10^-13 M
Explanation:
Since pH is the negative log of the hydronium concentration, we can solve for hydronium concentration and then plug in our given pH:
pH = -log[H⁺]
-pH = log[H⁺]
10^-pH = [H⁺]
Let's plug in our pH:
[H⁺] = 10^-pH = 10^-12.24 = 5.754 x 10^-13 M
acetanilide is soluble in warm water, but trans-cinnamic acid is not. suggest an explanantion for this looking at ratios of polar SA and total SA
Answers
Acetanilide has a higher ratio of polar surface area (SA) to total SA compared to trans-cinnamic acid, which allows it to form stronger interactions with water molecules and be more soluble.
Acetanilide and trans-cinnamic acid have different solubility behaviors in warm water due to their molecular structures and the relative ratios of their polar surface area (SA) to total SA.
Acetanilide contains an amide functional group (-CONH2), which contributes to its polar nature. The amide group has a partial positive charge on the carbon and a partial negative charge on the oxygen and nitrogen atoms. This polar group increases the ratio of polar SA to total SA in acetanilide, allowing it to form stronger hydrogen bonds and interact more favorably with water molecules, making it soluble in warm water. On the other hand, trans-cinnamic acid contains a carboxylic acid functional group (-COOH), which is also polar but to a lesser extent compared to the amide group. The lower polar SA to total SA ratio in trans-cinnamic acid results in weaker interactions with water molecules, leading to lower solubility in warm water.
Thus, the differences in the ratios of polar SA to total SA between acetanilide and trans-cinnamic acid explain their contrasting solubility behaviors in warm water.
learn more about trans-cinnamic acid here:
https://brainly.com/question/32797315
#SPJ11
HELP HElP HELp Please

Answers
Answer:
1- there are 933.5g in 2.578 mol of C7H5BiO4
2- the molar mass of C7H5Bi04 is 362.093g
Explanation:
To find grams you just want to convert moles into grams so:
2.578 mol • 362.093 (molar mass)
-—————- = 933.475754
1 mol
so 933.5g (for sigfigs)
finding the molar mass is actually very easy you just add up the molar mass from all the elements present in the compound (you can usually just round but for accuracy I’m using exact weights):
C - 7 (subscript) x 12.011 (molar mass) = 84.077 g
H - 5 (subscript) x 1.008 (molar mass) =
5.04 g
Bi - 1 (subscript) x 208.98 (molar mass) = 208.98 g
O - 4 (subscript) x 15.999 (molar mass) = 63.996 g
then just add them all:
84.077 g + 5.04 g + 208.98 g + 63.996 g = 362.093 g
so the molar mass of C7H5Bi04 is 362.093g
(you’ll notice that the molar mass here is the same as the one used in the equation from the first question)
hope this helps :)
An atom has an atomic mass of 223 and an atomic number of 87. Calculate the number of neutrons.
Answers
Answer:
136 neutrons
Explanation:
To find the number of neutrons of an atom, you subtract the atomic number (number of protons) from the atomic mass.
223 - 87 = 136
Benzene contains six carbon atoms bonded in a ring. Which best describes these bonds?
A six identical double bonds
B three identical single bonds alternating with three identical double bonds
C six identical bonds, each a hybrid between a single bond and a double bond
D six identical single bonds
Answers
B. Three identical single bonds alternating with three identical double bonds
The chemical bonding structure of benzene consists of six carbon atoms bonded together in a ring. In this structure, there are three single bonds and three double bonds, which are arranged in an alternating pattern around the ring. This bond arrangement is known as an aromatic bond and is characteristic of aromatic compounds such as benzene.
It is characterized by its high stability and low reactivity, which are due to the delocalization of the electrons in the ring.
Determine the molar mass of an unknown gas that has a volume of 72.5cm3 at a temperature of 68.0c, and a pressure of 99.0kpa, and a mass of 0.207g
Answers
The concept ideal gas equation is used here to determine the molar mass of the unknown gas. The ideal gas law is also known as the general gas equation and it is an equation of the state of a hypothetical ideal gas.
The ideal gas equation is developed as a result of the combination of the Boyles law, Charles's law and Avogadro's law. The ideal gas equation has several limitations.
The ideal gas law is:
PV = nRT
Number of moles = Given mass / Molar mass = m / M
99.0kpa = 0.977 atm
68.0 C = 341 K
72.5 cm³ = 0.0725 L
PV = m / M RT
0.977 × 0.0725 = 0.207 / M 0.0821 × 341
0.977 × 0.0725 = 5.795 / M
M = 82.78 g / mol
Thus the molar mass is 82.78 g / mol.
To know more about molar mass, visit;
https://brainly.com/question/30640134
#SPJ1
what is the molality of ions in a 0.507 m solution of alcl₃ assuming the compound dissociates completely?
Answers
The equation M = mol solute/kg solvent can be used to determine the molality of the ions in an AlCl3 solution at a concentration of 0.507 m, assuming that the molecule completely dissociates. The molality of the Cl and Al3+ ions in AlCl3 would both be 1.014 m as each mole is made up of two moles of Cl ions and one mole of Al3+ ions.
The amount of AlCl3 in moles must be known in order to compute the molality. The formula n = M x V, where M is the solution's molarity and V is the volume in litres, can be used to compute this. The amount of moles would be 0.507 moles for an AlCl3 solution in a 0.507 m solution. This indicates that the ions in the solution would have a molality of 1.014 m.
In conclusion, if the chemical entirely dissociates, the molality of ions in an AlCl3 solution at 0.507 m would be 1.014 m. This can be computed by counting the moles of AlCl3, then calculating the molality of the ions using the molality formula.
Know more about molality here
https://brainly.com/question/26921570#
#SPJ11
Which statement describes conditions in which a mineral can form?
A. Crystals of minerals dissolve in the groundwater in caves.
B. Molten materials are cooled in a metalworks factory.
C. Materials are mined from deposits deep underground,
D. Materials dissolved in seawater crystallize on an ocean bottom.
Answers
The statement describes conditions in which a mineral can form is "Crystals of minerals dissolve in the groundwater in caves."
What is Crystals?Atoms of the relatively similar element and atoms of other elements [such as silica (Si) and calcium (Ca)] can make up a crystal, and they are arranged in a predictable, repeating pattern.
What is minerals?A mineral would be an element as well as a chemical compound which has been produced as a result of geological activity and is often crystalline in nature.
The statement describes conditions in which a mineral can form as "Crystals of minerals dissolve in the groundwater in caves."
To know more about minerals and crystals.
https://brainly.com/question/18078524
#SPJ3
part a when a ketone and its enol are in equilibrium, under most conditions the concentration of the enol is ________ the concentration of the ketone.
Answers
Answer:
Much lower than ketone is more stable than enol. N, 4-Dimethylpent -4-en-2-Amine (NH_3 protonated in acidic protoned in acidic conditions) d. [Proton cannot re extracted from OH in acidic conditions to firm O^(-)]
What needs to be known to determine the critical values for an independent-samples t test?
- the sample size of the power analysis
- degrees of freedom for the smallest group
- total degrees of freedom
- degrees of freedom for the largest group
Answers
The critical value for an independent-samples t-test is total degrees of freedom. Option C.
Each topic must belong to only one group. There is no relationship between observations in each group. There are no significant outliers in the two groups. The independent-sample t-test is appropriate when the researcher wants to know whether the means of her two population groups differ.
Independent samples t-test tells whether the organization has a statistical difference between her two sets of categories or items, and if there is a statistical difference, the difference is significant. when the observations in each group are independent of the observations in the other group increase.
Learn more about The critical value here:- https://brainly.com/question/14040224
#SPJ4
What happens to the energy as metal parts cool
Answers
Answer: it transfers out of the metal the heat stops and the energy is absorbed by other things
Explanation:
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
What is a map? Explain it in a sentence.
PLEASE HELPP
Answers
Answer:
A map is a visual representation of all land
Explanation:
im smart
Answer: a map is a peice of paper or plastic that shows you the world and the contenents and the state and the countries ,.
Explanation:
How did alchemists contribute to science ?
Answers
Answer:
Alchemy is an ancient practice shrouded in mystery and secrecy. Its practitioners mainly sought to turn lead into gold.
Explanation:

The heat of reaction for the combustion of 1 mole of ethyl alcohol is -9. 50x 10^2 kJ. How much heat is produced when 11. 5g of alcohol is burned?
Answers
When 11.5g of ethyl alcohol is burned, approximately -2.37 x 10^2 kJ of heat is produced. The negative sign indicates that the reaction releases heat, which is consistent with the exothermic nature of combustion reactions.
The heat of reaction for the combustion of 1 mole of ethyl alcohol is -9.50 x 10^2 kJ. This means that when 1 mole of ethyl alcohol is burned, 9.50 x 10^2 kJ of heat is released.
To calculate how much heat is produced when 11.5g of alcohol is burned, we first need to determine the number of moles of alcohol in 11.5g. We can do this using the molar mass of ethyl alcohol, which is 46.07 g/mol:
moles of alcohol = mass of alcohol / molar mass
moles of alcohol = 11.5 g / 46.07 g/mol
moles of alcohol = 0.2496 mol
Now that we know the number of moles of alcohol, we can use the heat of reaction to calculate the amount of heat produced:
heat produced = moles of alcohol x heat of reaction
heat produced = 0.2496 mol x (-9.50 x 10^2 kJ/mol)
heat produced = -2.37 x 10^2 kJ
Learn more about alcohol here:
https://brainly.com/question/29153788
#SPJ11
deposits of natural gas are most numerous in ________.
Answers
Deposits of natural gas are most numerous in sedimentary basins.
Sedimentary rock layers that have accumulated over millions of years define sedimentary basins as geological formations. Natural gas can be generated and stored under optimal conditions in these basins. The remains of ancient marine plants and animals can be found in organic-rich sedimentary rocks, such as shale.
Organic matter is slowly converted by heat and pressure into hydrocarbons, including natural gas. In sedimentary basins, there may be natural gas trapped in porous and permeable reservoir rocks such as sandstone or limestone. Finding and extracting natural gas from these many sedimentary basins is a main goal of exploration and production efforts around the world.
Learn more about sedimentary basins, here:
https://brainly.com/question/33181953
#SPJ4
The most numerous deposits of natural gas are found in the Middle East, Russia, the United States, Canada, and China.
Natural gas deposits are found in various parts of the world, but some regions are known for their abundant reserves. The most numerous deposits of natural gas can be found in the Middle East, Russia, the United States, Canada, and China.
The Middle East, including countries like Saudi Arabia, Iran, and Qatar, is known for its vast natural gas reserves. Russia, as one of the largest producers of natural gas, also has significant deposits. The United States has seen a boom in natural gas production in recent years, particularly from shale gas formations. Canada is another country with substantial natural gas reserves, mainly located in the western provinces. China, with its growing energy demands, has also been actively exploring and developing its natural gas resources.
These regions have favorable geological conditions and extensive exploration and production activities, which contribute to the abundance of natural gas deposits.
Learn more:
About deposits here:
https://brainly.com/question/2507231
#SPJ11
why does potassium explode when exposed to water?
Answers
Answer:
The highly unstable pure sodium or potassium wants to lose an electron and this splits the water atom, producing a negatively charged hydroxide ion and hydrogen and forming an explosive gas that ignites.
Explanation:
If Steve throws the football 50 meters in 5 seconds, what is the average speed of the football?
Answers
Answer:
10 meters per second
Explanation:
What is the type of pollutant in waterways which can be toxic at high levels and is
present in the river mentioned in question 1?
Olead
OE. coli
Oammonial
Onitrates
Answers
The type of pollutant in waterways which can be toxic at high levels is nitrates.
What is pollutant?Pollutant is a substance that is introduced into the environment and has negative effects on living organisms and the environment. It can be either natural or man-made and can include air pollutants such as carbon dioxide, sulfur dioxide, nitrogen oxides, and particulate matter, as well as water pollutants such as nutrients, heavy metals, and toxic chemicals.
Nitrates are nitrogen-containing compounds and are often found in fertilizers, wastewater and agricultural runoff. Nitrates can lead to oxygen depletion in water and can be harmful to aquatic life and humans if consumed in high amounts. The River Avon in Warwickshire is known to have high levels of nitrates due to agricultural runoff.
To learn more about pollutant
https://brainly.com/question/1347424
#SPJ1
For the following equations which define the behaviour of the technology level:
In At = A + gt +At
At = rhoAA~t−1+ϵA,t,−1
a) Express lnA1, lnA2, and lnA3 in terms of lnA0, εA,1, εA,2, and εA,3.
b) Calculate the expected values of lnA1, lnA2 and lnA3 taking as constants , lnA0, rhoA and g.
Answers
To express lnA1, lnA2, and lnA3 in terms of lnA0, εA,1, εA,2, and εA,3, we can use the given equations: From the equation At = A + gt + At, we can rewrite it as At - gt = A + At. Taking the natural logarithm (ln) of both sides, we have ln(At - gt) = ln(A + At).
Similarly, from the equation At = rhoAA~t−1 + ϵA,t,−1, we can rewrite it as At - rhoAA~t−1 = ϵA,t,−1. Taking the natural logarithm (ln) of both sides, we have ln(At - rhoAA~t−1) = ln(ϵA,t,−1). Now, let's express lnA1, lnA2, and lnA3 in terms of ln A0, εA,1, εA,2, and εA,3. Expressing lnA1:
- From equation 1, we have ln(A1 - g1t) = ln(A0 + A1).
Rearranging the equation, we get ln(A1 - g1t) - ln(A1) = ln(A0).
- From equation 2, we have ln(A1 - rhoAA~1−1) = ln(εA,1).
Rearranging the equation, we get ln(A1 - rhoAA~1−1) - ln(A1) = ln(εA,1).
Therefore, lnA1 = ln(A0) + ln(εA,1).
Calculating the expected values of lnA1, lnA2, and lnA3: - Taking the expected value (E) of equation 1, we have E[ln(A1 - g1t)] = E[ln(A0 + A1)]. Since g1t is constant, we can write it as E[ln(A1)] - g1t = ln(A0 + E[A1]).
Rearranging the equation, we get E[ln(A1)] = ln(A0 + E[A1]) + g1t.
- Taking the expected value (E) of equation 2, we have E[ln(A1 - rhoAA~1−1)] = E[ln(εA,1)]. Since rhoAA~1−1 is constant, we can write it as E[ln(A1)] - rhoAE[A~1−1] = ln(εA,1).
Rearranging the equation, we get E[ln(A1)] = ln(εA,1) + rhoAE[A~1−1].
Therefore, the expected value of lnA1 is given by E[lnA1] = ln(A0 + E[A1]) + g1t = ln(εA,1) + rhoAE[A~1−1]. Similarly, we can calculate the expected values of lnA2 and lnA3 using the corresponding equations and constants.
To know more about equations visit:
https://brainly.com/question/14686792
#SPJ11
determine the concentration of nh3(aq) that is required to dissolve agcl
Answers
The concentration of NH3(aq) that is required to dissolve AgCl is 1.0 × 10−4 M.
When AgCl is dissolved in a solution of NH3(aq), the following reaction takes place:
AgCl(s) + 2NH3(aq) ⟶ Ag(NH3)2+(aq) + Cl−(aq)
Since NH3(aq) is acting as a ligand to form a complex ion, the equilibrium constant expression for the reaction is written as:
Kf = [Ag(NH3)2+] [Cl−]/ [AgCl]
The solubility product constant expression (Ksp) for AgCl is written as:
Ksp = [Ag+][Cl−]
Since AgCl is a sparingly soluble salt, its solubility is low, so its concentration can be assumed to be negligible. Therefore, Ksp = [Ag+][Cl−] ≈ [Ag+] [NH3]2
Kf can be calculated from standard tables and is equal to 1.5 × 107 M−1.
Substituting the values of Kf and Ksp in the above equation:
Kf = [Ag(NH3)2+] [Cl−]/ [AgCl]
1.5 × 107 M−1 = [Ag(NH3)2+] [1.0 × 10−5 M]/ [AgCl]
Simplifying, [Ag(NH3)2+] = 1.5 × 10−4 M
Therefore, the concentration of NH3(aq) required to dissolve AgCl is 1.0 × 10−4 M.
Learn more about solubility product here:
https://brainly.com/question/32662127
#SPJ11
what is the compound name for Cu(OH)2
Answers
Answer:
Copper hydroxide is the correct answer.
Explanation:
Hope this helped Mark BRAINLIEST!!!
copper(||)hydroxide
Part 1: Predict which compound in each pair is more acidic. Explain your answers
. a) cyclopentanol or 3-clorophenol
b) cyclohexanol or cyclohexantiol
c) cyclohexanol or cyclohexanecarboxylic acid
d) 2,2-dichlorobutan-1-ol or butan-1-ol
Part 2: Predict which compound in each group is more soluble in water. Explain your answers.
a) butan-1-ol, pentan-1-ol or propan-2-ol
b) chlorocyclohexane, cyclohexanol or cyclohexane-1,2-diol
c) phenol, cyclohexanol or 4-methylcyclohexanol
Answers
(1a) 3-Chlorophenol is more acidic than cyclopentanol (chlorine atom electron-withdrawal). (1b) Cyclohexanecarboxylic acid is more acidic than cyclohexanol (stronger carboxylic acid group).
(2a) Propan-2-ol is more soluble in water than butan-1-ol and pentan-1-ol (hydrogen bonding ability). (2b) Cyclohexanol is more soluble in water than chlorocyclohexane (hydroxyl group enables hydrogen bonding).
Part 1: Comparing Acidic Strength
a) 3-chlorophenol is more acidic than cyclopentanol. This is because the presence of a chlorine atom in 3-chlorophenol can stabilize the negative charge on the phenoxide ion through inductive and resonance effects, making it more stable and easier to form.
b) Cyclohexanecarboxylic acid is more acidic than cyclohexanol. The carboxylic acid group (-COOH) is a stronger acid functional group compared to the hydroxyl group (-OH) present in cyclohexanol.
c) 2,2-dichlorobutan-1-ol is more acidic than butan-1-ol. The presence of the electron-withdrawing chlorine atoms in 2,2-dichlorobutan-1-ol enhances the acidity by stabilizing the negative charge on the alkoxide ion formed upon deprotonation.
d) Cyclohexanecarboxylic acid is more acidic than cyclohexanol. The carboxylic acid group (-COOH) is a stronger acid functional group compared to the hydroxyl group (-OH) present in cyclohexanol.
Part 2: Comparing Solubility in Water
a) Propan-2-ol is more soluble in water than butan-1-ol and pentan-1-ol. Propan-2-ol has a hydroxyl group (-OH) that can form hydrogen bonds with water molecules, increasing its solubility.
b) Cyclohexanol is more soluble in water than chlorocyclohexane. The presence of the hydroxyl group in cyclohexanol allows for hydrogen bonding with water molecules, enhancing its solubility. Chlorocyclohexane, on the other hand, is nonpolar and lacks the ability to form significant hydrogen bonds with water.
c) Cyclohexanol is more soluble in water than phenol and 4-methylcyclohexanol. Both cyclohexanol and phenol can form hydrogen bonds with water, but phenol's aromatic ring reduces its solubility. 4-methylcyclohexanol is also less soluble than cyclohexanol due to the steric hindrance from the methyl group, which disrupts hydrogen bonding.
To know more about the aromatic ring refer here,
https://brainly.com/question/32170261#
#SPJ11
When a chemical reaction does not occur, what happens to the atoms of the two substances?
please make it sound like a 7th grdaer would say! <3
Answers
Answer:
In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed. In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
consider marking me brainlist :)
In which situation are unbalanced forces acting on an object?
Two people stand on opposite sides of a large tire. Both people pull the tire with equal force.
Two people stand on opposite sides of a large tire. Both people push the tire with equal force.
Two people stand on the same side of a large tire. Both people pull the tire with equal force.
Two people stand on the same side of a large tire. One person pushes the tire and the other pulls the tire with equal force.
Answers
The situation in which two people stand on the same side of a large tire, both people pull the tire with equal force is creating an unbalanced force. The force from the opposite side too acts on the tire and make the displacement.
What is force ?Force is an external agent acting on a body to change its motion or to deform it. The net force acting on the body depends on the direction and magnitude of all the force acting on it.
If two equal forces acts on the body from the same side they add up together and the net force is their sum. If the forces are acting from the opposite sides, they will cancel each other and is said to be balanced.
Here, two forces from the side is not balanced by a force from the opposite side in case 3 and make a displacement. Thus, the option 3 is correct.
Find more on unbalanced forces:
https://brainly.com/question/29769471
#SPJ1
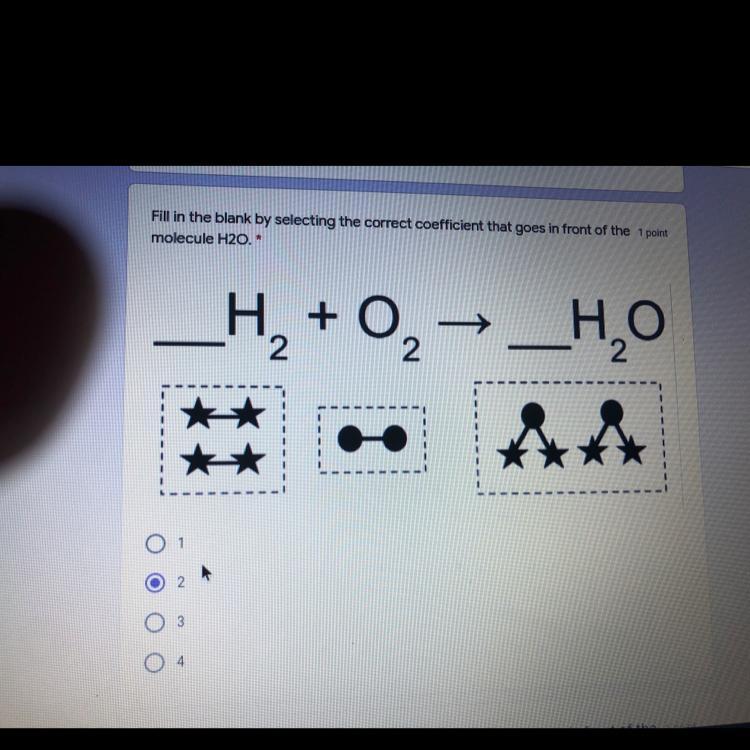
The stars are H and the circles are O by the way
Answers
Answer:2 is correct
Explanation: