What would make oppositely charged objects attract each other more?
O increasing the positive charge of the positively charged object and increasing the negative charge of the
negatively charged object
O decreasing the positive charge of the positively charged object and decreasing the negative charge of th
negatively charged object
increasing the distance between the positively charged object and the negatively charged object
O maintaining the distance between the positively charged object and the negatively charged object
Answers
Answer:
The correct answer is A. increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged object
I hope this helped :D
Related Questions
What is 1.5 converted
Answers
Answer:
3/5
Explanation:
Think about it: Gold is one of the densest substances known, with a density of 19.3 g/cm3. If the gold in the crown was mixed with a less-valuable metal like bronze or copper, how would that affect its density?
Answers
Answer:
If gold has a rare, high density then it would sink quickly. If mixed substances that are less-valued, are added to the gold crown (remember that gold is rare and very dense which makes it special) then we can assume the cheap substances are less dense, thus making the crown FLOAT more rather than sink (I say more, because unless the crown was extremely mixed with cheap material then it could possibly float but it depends on how much is in the crown). Summary: The crown would either be lighter and float, or barely be sunken due to the less-dense substance.
*Hint to think about: People consider cheaper things lighter such as plastic ring/less dense for example, compared to a silver ring which is heavier/more dense (btw heavy does not always mean high density, it depends on the liquid density )
Hope this actually helps!
Explanation:
High-Desnity=Sink
Low Density=Float
According to the law of conservation of energy, in theory a bouncy ball should not stop bouncing. But however, we know that it eventually stops. Where does the energy go? Used reasoning and evidence to explain
Answers
A bouncing ball gradually stops bouncing because its energy is converted to heat energy.
According to the law of conservation of energy, energy is neither created nor destroyed but can be transformed from one form to another.
When a ball is bouncing up and down, we notice that the ball will slow down gradually. This is because, the energy in the bouncing ball is transferred to the small air molecules inside the ball as heat. Hence, the ball looses energy consistently until it comes to a stop.
Learn more: https://brainly.com/question/1527403
A 1.555 g sample of baking soda decomposes with heat to produce 0.991 g Na2CO3. What is the percent yield of sodium carbonate, Na2CO3?
Answers
Answer:
Explanation:
Baking soda decomposes to produce sodium carbonate .
2NaHCO₃ = Na₂CO₃ + CO₂ + H₂O.
2 x 84 g 106 + 10 x 18 g
Molecular mass of Na₂CO₃ .10H₂O = 286
168 g baking soda produces 286 g sodium carbonate
1.555g baking soda will produce 286 x 1.555 / 168
= 2.647 g
Percentage yield = actual yield / theoretical yield x 100
= ( .991 /2.647 ) x 100
= 37.43 %.
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
A compound is found to contain 9.227 % boron and 90.77 % chlorine by mass. What is the empirical formula for this compound?
Answers
Assuming a 100 g sample of the compound, we can convert the mass percentages to masses in grams:
- 9.227 g B
- 90.77 g Cl
Next, we need to convert these masses to moles using the atomic masses of the elements:
- B: 10.81 g/mol
- Cl: 35.45 g/mol
- 9.227 g B ÷ 10.81 g/mol = 0.853 mol B
- 90.77 g Cl ÷ 35.45 g/mol = 2.562 mol Cl
Now we need to divide both mole values by the smaller of the two, which is 0.853 mol:
- 0.853 mol B ÷ 0.853 mol = 1.000 mol B
- 2.562 mol Cl ÷ 0.853 mol = 3.000 mol Cl
This gives us a B:Cl ratio of 1:3. The empirical formula for the compound is therefore BCl3.
Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Explanation:
Mass of boron and chlorine is 9.224% and 90.74%
In the Periodic Table, which of the highlighted elements has the greatest number of protons?
A Fluorine, F
B Iron, Fe
C Calcium, Ca
D Aluminum, AI

Answers
Answer:
since the modern periodic table is made in ascending order by the atomic number fluorine has the most protons.
The highlighted element that has the greatest number of protons is Iron, Fe. Thus, the correct option for this question is B.
What do you mean by Protons?Protons may be defined as a type of subatomic particles which are generally present in the centermost part of an atom known as the nucleus along with neutrons. These subatomic particles are positively charged in nature. Ernest Rutherford was known to discover these particles.
According to the question, the number of protons is generally governed by an atomic number of a chemical element. For example, fluorine has an atomic number of 9. It has the same number of protons in its nucleus. The number of protons in Iron is 26, while the number of protons in Calcium is 20. And the number of protons in aluminum is 13.
Therefore, an iron is the highlighted element that has the greatest number of protons. Thus, the correct option for this question is B.
To learn more about Protons and atomic numbers, refer to the link:
https://brainly.com/question/1805828
#SPJ2
I need help. Look at the pictures.



Answers
Answer:
sorry I would help but I havnt started learning about that yet
Explanation:
I just don't know
in the ideal gas law which variable represents the gas constant?
a: T
b: R
c: n
d: V
e: P
Answers
Explanation:
It is represented using the ideal gas equation , or PV = nRT, where P is the pressure in atmospheres, V is the volume in liters, n represents the quantity of particles in the container, T represents the temperature in Kelvin, and R is the ideal gas constant equal to 0.0821 liters atmospheres per moles Kelvin.
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676
Draw a structural formula for the major product of the reaction shown.
Answers
Draw a structural formula for the major product of the reaction shown:
The structural formula for the major product (2-butene) of the given reaction is as follows:$$\ce{CH3CH2CH=CH2}$$
The given reaction is an acid-catalyzed dehydration reaction.
During the reaction, the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the reactant alcohol (2-butanol) undergo dehydration (loss of water) to form an alkene (2-butene) as the major product.
The reaction is shown below:$$\ce{CH3CH2CH2CH2OH + H2SO4 ->[\Delta] CH3CH2CH=CH2 + H2O}$$To draw the structural formula for the major product of the given reaction, we need to consider the following points:
1. The reactant alcohol (2-butanol) is a four-carbon alcohol with the hydroxyl group (OH) attached to the second carbon atom (C2) of the chain.
2. The product alkene (2-butene) will be a four-carbon alkene with a double bond between the second and third carbon atoms (C2 and C3) of the chain.
The other two carbon atoms will have a single bond with the adjacent carbon atoms and a hydrogen atom each attached to them.
3. The major product will be formed via the elimination of water (dehydration) between the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the second carbon atom (C2) of the reactant alcohol (2-butanol).
4. The acid catalyst (H2SO4) does not participate in the reaction and remains unchanged. It only facilitates the formation of the alkene by providing a proton (H+) to the hydroxyl group (OH) and a medium for the elimination of water.
For more such questions on alkene
https://brainly.com/question/27704061
#SPJ8
which probing question lies within the scope of physics?
Answers
Physics is a vast field that addresses a wide range of questions about the nature of the physical world. Probing questions can help to explore this field and encourage critical thinking and deep exploration of its topics.
Probing questions are open-ended questions asked to gather information, encourage critical thinking and deep exploration of a particular topic. Physics is a natural science that studies matter and its motion through space-time. It is a branch of science that deals with the fundamental nature of the universe and seeks to explain how and why objects behave as they do in the physical world.The following are some examples of probing questions within the scope of physics:What is the nature of light-The nature of light is an important topic within the scope of physics. It refers to the dual nature of light, as both a wave and a particle. Light behaves as a wave when it is traveling through space and as a particle when it is interacting with matter.How do magnets work-Magnets are a common object in the world around us, and they have a broad range of applications. They work by producing a magnetic field, which can attract or repel other magnetic objects. This topic lies within the scope of physics.What is the relationship between energy and matter-Energy and matter are two fundamental concepts in physics. The relationship between them is described by Einstein's famous equation E=mc2, which states that matter and energy are two forms of the same thing and are interchangeable. The study of the relationship between energy and matter lies within the scope of physics.What is the nature of the universe?The study of the universe's nature is one of the most significant topics within the scope of physics. This question addresses the origins and properties of the universe, its components, and the laws that govern its behavior.
for such more questions on physical
https://brainly.com/question/1079154
#SPJ8
Which of the following chemical formulas represents a common acid?
*
NaOH
KI
HCI
H20
Answers
Question 18 (1 point)
What is the pH of a buffer that is 0.086 M HF and 0.386 M LiF? The K₂ for HF is 3.5
x 10-4.
Your Answer:
Answer
Answers
The pH of a buffer that is 0.086 M HF and 0.386 M LiF when the K₂ for HF is 3.5x 10-4 is 2.802
The pH of a buffer solution is given by Henderson Hasselback equation.
pH=pKa+log[A-]/[HA]
Substituting the values, we get
pH=-log(3.5x10^-4)+log(0.086/0.386)
pH=-log(3.5x10^-4)-0.653
pH=3.455-0.653
pH=2.802
pH is the potenz of hydronium ion.It tells the acidic strength of a compound.Buffer solution resists the change in pH on the addition of weak acid or base and conjugate of weak acid or base.It is used in acid-base chemistry.Buffer solutions are used for keeping the pH at a constant value.To learn more about buffer solutions visit:
brainly.com/question/24262133
#SPJ1
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
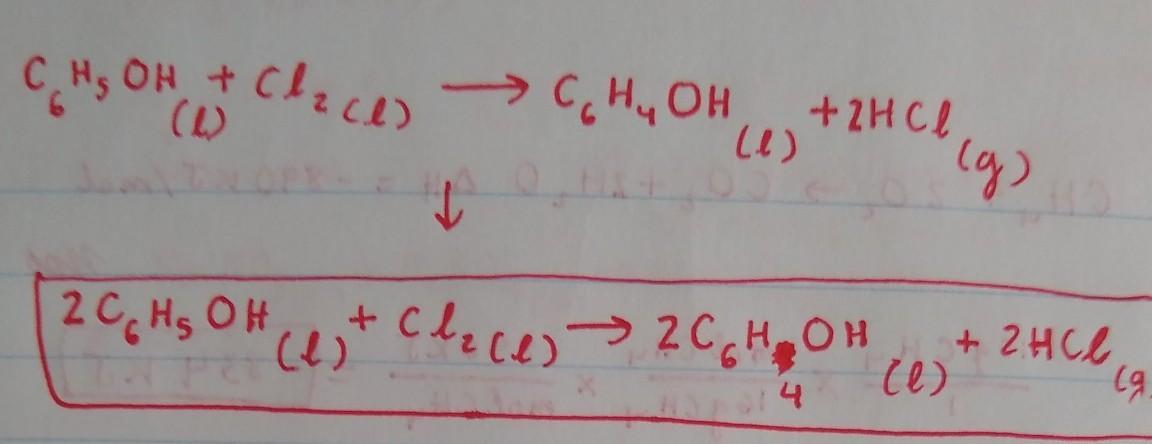
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
Give the symbol and name for the ion with 34 protons and 36 electrons.
Answers
Answer:
That element is selenium, Se
The hydrogen atom is not actually electronegative enough to form bonds to xenon. Were the xenon-hydrogen bond to exist, what would be the structure of XeH4
Answers
Answer:
Square planar
Explanation:
There are six electron domains in XeH4. Four of them are bond pairs while two of them are lone pairs.
When a central atom has six electron pairs, the bond pairs occupy the corners of a square while the two lone pairs are found above and below the plane of the square.
This is generally know as the square planar molecular geometry
Sometimes pieces of a marshmallow fall off when browned do you have to include the pieces that fall off when determining the mass before and after?
Answers
The marshmallow's dark spots occasionally have a tendency to come off. This implies that the system does not have to take the pieces that fell into account when calculating the mass before and after.
What causes marshmallows to get stickier as they heat up?Explain your response using chemistry. The marshmallow's sugar matrix and air bubbles help keep the gelatin inside the marshmallow in check. The air bubbles in the marshmallow burst, allowing the gelatin to move freely, when heat or moisture is added.
What substances does a marshmallow contain?A regular marshmallow has air, corn syrup, sugar, and gelatin. All done. Richard Hartel, a food engineer at the University of Wisconsin-Madison, claims that a marshmallow is essentially a foam that has gelatin added to solidify it. in the froth on marshmallows is comprised of air that has been suspended in a sugary liquid.
To know more about marshmallow visit:-
https://brainly.com/question/3321108
#SPJ13
How many moles of carbon dioxide gas occupy a volume of
81.3 L at 204 kPa and a temperature of 95.0 °C?
mol CO2
Answers
Answer:
5.42
Explanation:
I used a calculator
Answer: 5.42 mol CO2
Explanation: Uses idea gas law
If 120.4 grams of reactant completely breaks down (decomposes) to produce 106.4 grams of chlorine. What mass of nitrogen gas could be expected? *
Answers
Answer:
\(m_{nitrogen}=14g\)
Explanation:
Hello,
In this case, since we are talking about a chemical reaction in which a compound having nitrogen and chlorine is decomposed into chlorine and nitrogen, we must remember that the law of conservation of mass must be obeyed, for that reason, we notice that the mass of the whole reactants must equal the mass of the whole products, as shown below:
\(m_{reactants}=m_{products}\)
Next, we know there is only one single reactant and products are constituted by both chlorine and nitrogen:
\(m_{reactant}=m_{chlorine}+m_{nitrogen}\)
In such a way, we can compute the mass of nitrogen as shown below:
\(m_{nitrogen}=m_{reactant}-m_{chlorine}=120.4g-106.4g\\\\m_{nitrogen}=14g\)
Best regards.
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

a. Analysis of the potassium ion content in a food sample yielded the following data: % K: 3.09, 4, 2.775, 2.5, 3.80 Calculate the standard deviation of the sample. Show all calculations and indicate the answer to the correct amount of significant figures.
Answers
The standard deviation of the sample is 0.579, rounded to the correct number of significant figures.
To calculate the standard deviation of the sample, we need to follow these steps:
Calculate the mean (average) of the data set.To find the mean, we sum up all the data points and divide by the number of data points. Let's calculate it:
(3.09 + 4 + 2.775 + 2.5 + 3.80) / 5 = 16.165 / 5 = 3.233
Subtract the mean from each data point.To do this, we subtract the mean (3.233) from each data point and square the result:
(3.09 - 3.233)^2 = 0.020049
(4 - 3.233)^2 = 0.586489
(2.775 - 3.233)^2 = 0.209025
(2.5 - 3.233)^2 = 0.537289
(3.80 - 3.233)^2 = 0.323329
Calculate the variance.To find the variance, we sum up the squared differences from step 2 and divide by the number of data points:
(0.020049 + 0.586489 + 0.209025 + 0.537289 + 0.323329) / 5 = 1.676181 / 5 = 0.3352362
Take the square root of the variance to get the standard deviation.√0.3352362 = 0.579 (rounded to three significant figures)
For such more questions on mean
https://brainly.com/question/1668275
#SPJ8
A propane tank when first filled reads 175. psi. After 1 month of use, the propane tank reads 81. psi.
Note: Reference the Conversion factors for non-SI units table for additional information.
Part 1 of 2
Convert the tank pressure when first filled to mmHg. Be sure your answer has the correct number of significant figures.
m
175. ps1=
mmHg
Answers
Answer:
After 1 month of use, the propane tank reads 81
Explanation:
'Which of the following best describes cis-trans isomers? See Concept 4.2 (Page 60)View Available Hint(s)They differ in the arrangement of covalent bonds and in covalent partners.They are long chains of hydrogen and carbon atoms.They are mirror images of each other.They have the same number of atoms of the same elements but different structures.They differ in their spatial arrangement around inflexible double bonds
Answers
The best description of cis-trans isomers is that they differ in their spatial arrangement around inflexible double bonds. Option E is correct.
Specifically, cis-trans isomers are a type of stereoisomers that have the same molecular formula and the same covalent bonds but differ in the spatial arrangement of their atoms due to the inflexibility of a double bond. In cis isomers, the two substituents on each carbon atom are on the same side of the double bond, while in trans isomers, the two substituents on each carbon atom are on opposite sides of the double bond.
This results in different physical and chemical properties for the two isomers. For example, cis and trans isomers of some compounds may have different boiling points, melting points, and reactivities. Cis-trans isomerism is an important concept in organic chemistry as it affects the properties and behavior of molecules and can have significant implications for their biological activity, environmental fate, and industrial applications. Option E is correct.
To know more about the Covalent bonds, here
https://brainly.com/question/13130950
#SPJ1
Why do scientists look for patterns in the world?
A. Patterns never change, no matter what.
B. Patterns can help explain observations.
O C. Patterns are easy for scientists to detect.
D. Patterns are all the same, through all time.
Answers
Answer:
B. Patterns can help explain observations.
Explanation:
Hope this helps
Answer:
B. Patterns can help explain observations.
Explanation:
a p e x 2021 :) Have a nice day everyone!
In the light equation what does A represent
O the wavelength.
O the frequency.
O the speed of light.
O Planck's constant.
Answers
there is no A in a light equation
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
Write symbols for four elements that may have ions with the following electron configuration: 1s22s22p6. (Include the charge of the ion with the symbol. For example, if "Ca2+" is the correct answer enter "Ca2+" in the box.) Write the most positive ion first and the most negative ion last.
Answers
The electron configuration 1s22s22p6 corresponds to the noble gas neon (Ne), which has a completely filled valence shell.
What is Electric Configuration?
The placement of electrons around the nucleus of a specific atom or molecule is known as its electronic configuration.
Protons, neutrons, and electrons are the minuscule components that make up an atom. There are the same number of protons and electrons in a neutral atom. The quantity and location of an atom's electrons are revealed by its electronic configuration.
We say that electrons orbit the nucleus of an atom, like the rings of Saturn orbit the planet. Electrons move in orbitals that can accommodate a specific number of electrons as they circle the nucleus.
Four elements that may have ions with this electron configuration are:
Fluorine (F-) - gains one electron to achieve a noble gas configuration (1s22s22p6)
Oxygen (O2-) - gains two electrons to achieve a noble gas configuration (1s22s22p6)
Sodium (Na+) - loses one electron from the valence shell to achieve a noble gas configuration (1s22s22p6)
Magnesium (Mg2+) - loses two electrons from the valence shell to achieve a noble gas configuration (1s22s22p6)
Therefore, the symbols for these ions are:
Na+, Mg2+, F-, O2-.
Learn more about Electronic Configuration from given link
https://brainly.com/question/26084288
#SPJ4