What type of ions do nonmetals naturally form?
Answers
Answer:
Negative Ions
Explanation:
Nonmetals naturally form Negative Ions by gaining electrons to fill the valence shell
Related Questions
Which phase of matter is the least common on Earth?
A. Gases
B. Liquids
C. Solids
D. Plasma
Answers
Answer:
the answer is....... D. Plasma
Answer:
Plasma
Explanation:
Got it right
silver nitrate decomposed when it is strongly
heated according to equation below :
2AgNO3 ->2Ag + 2NO2 + O2
In an experiment, a student heated 85g of
silver nitrate.Calculate the mass of silver
produced at room condition. (Relative atomic
mass : N = 14,0 =16, Ag =108)
Answers
The mass of silver produced at room conditions is approximately 54.324 grams.
To calculate the mass of silver produced, we need to determine the number of moles of silver nitrate used in the reaction, and then use the stoichiometry of the balanced equation to find the number of moles of silver produced.
First, let's calculate the number of moles of silver nitrate used:
Molar mass of AgNO3 = 108 + 14 + (3 * 16) = 169 g/mol
Number of moles of AgNO3 = Mass of AgNO3 / Molar mass of AgNO3
Number of moles of AgNO3 = 85 g / 169 g/mol
Number of moles of AgNO3 ≈ 0.503 moles
According to the balanced equation, 2 moles of AgNO3 produce 2 moles of Ag.
Since the molar ratio is 1:1, the number of moles of Ag produced is also 0.503 moles.
Now, let's calculate the mass of silver produced:
Molar mass of Ag = 108 g/mol
Mass of Ag = Number of moles of Ag * Molar mass of Ag
Mass of Ag = 0.503 moles * 108 g/mol
Mass of Ag ≈ 54.324 g
Explain why the molecules were moving that way after energy was transferred out of them.
Answers
The molecules were moving that way after energy was transferred out of them due to the principles of thermodynamics.
When energy is transferred out of molecules, their movement is governed by the principles of thermodynamics. The movement of molecules is primarily influenced by two key factors: temperature and entropy.
Temperature is a measure of the average kinetic energy of the molecules. When energy is transferred out of the molecules, their kinetic energy decreases, causing the molecules to slow down. As a result, the molecules exhibit less random motion and have lower velocities.
Entropy, on the other hand, is a measure of the randomness or disorder within a system. When energy is transferred out of the molecules, their overall level of disorder decreases. This reduction in disorder tends to align the molecules in a more ordered or structured manner, such as in a solid state. As a result, the molecules may undergo a decrease in random motion and tend to occupy more confined or specific positions.
Learn more about the principles of thermodynamics
brainly.com/question/33942666
#SPJ11
A label on an empty sample container reads 12. 00 g. you add in a sample of a compound and mass the sample container obtaining 15. 5465 g. what should the mass of the sample be reported as?
Answers
The mass of the substance is the quantitative measure of the physical object. The mass of the sample must be reported as 3.5465 g. Thus, option e is correct.
What is mass?The mass of the substance is the quantity of matter that the sample weighs and is reflected in grams or kilograms.
Given,
Mass of empty sample container = 12.00 g
Mass of sample + empty container = 15.5465 g
The mass of the sample will be given as,
mass of sample = (Mass of sample + empty container) - ( Mass of empty container)
= (15.5465 g - 12.00g)
=3.5465 g
Therefore, 3.5465 gm is the mass of the sample, and the correct option is e.
Your question is incomplete, but most probably your full question was, A label on an empty sample container reads 12.00 g. you add in a sample of a compound and the mass of the sample container obtaining 15.5465 g. what should the mass of the sample be reported as?
3.546 g3.54 g4 g4.0 g3.5465 gLearn more about mass here:
https://brainly.com/question/17270802
#SPJ4
help!!!!?????????? plssssssss

Answers
Answer: it’s b)
Explanation: that’s the only difference that is listed
Answer:
I think it is B
Explanation:
Propose which one of the following reactions is more likely to occur through SN1
mechanism?
A. (CH3)2CH − Cl
CCl4/NaOH
→ CH2)2 CH − OH ⃝
B. C2H5Cl
NaOH/H2O
→ C2H5 − OH ⃝
C. (CH3)3 CCl
H2O
NaOH
→ (CH3)3C − OH ⃝
D. (��3)3� − ��
���4/����
→ (��3)3���
Answers
Based on carbocation stability, reaction C is more likely to occur through the SN1 mechanism. Option C
The SN1 mechanism is a nucleophilic substitution reaction that proceeds through a two-step process. In the first step, the leaving group departs, forming a carbocation intermediate. In the second step, the nucleophile attacks the carbocation, resulting in the formation of the substitution product.
To determine which reaction is more likely to occur through the SN1 mechanism, we need to assess the stability of the carbocation intermediate formed during the reaction. Carbocation stability is influenced by the number of alkyl groups attached to the positively charged carbon atom. The greater the number of alkyl groups, the more stable the carbocation.
Let's analyze the given reactions:
A. (CH3)2CH − Cl → (CH3)2CH − OH
B. C2H5Cl → C2H5 − OH
C. (CH3)3CCl → (CH3)3C − OH
In reaction A, we have a secondary alkyl halide ((CH3)2CH−Cl), which forms a secondary carbocation during the reaction. This carbocation is relatively stable due to the two methyl groups attached to the positively charged carbon atom.
In reaction B, we have a primary alkyl halide (C2H5Cl), which forms a primary carbocation. Primary carbocations are less stable compared to secondary or tertiary carbocations.
In reaction C, we have a tertiary alkyl halide ((CH3)3CCl), which forms a highly stable tertiary carbocation. Tertiary carbocations are the most stable due to the presence of three methyl groups.
Option C
For more such questions on carbocation visit:
https://brainly.com/question/11486868
#SPJ8
Under which set of conditions would H₂ (g) be the most dissolved in H₂O(l)?
101.3 kPa and 75°C
120 kPa and 25°C
101.3 kPa and 25°C
120 kPa and 75°C
Answers
The most dissolved H₂ (g) in H₂O (l) would occur under 101.3 kPa and 75°C.
The attraction between an electronegative atom serving as the hydrogen bond acceptor and a hydrogen atom covalently bonded to a more electronegative "donor" atom or group (Dn) is known as a hydrogen bond, or H-bond (Ac).Under 101.3 kPa and 75 °C, the maximum dissolved H2 (g) in H2O (l) would be present.At higher temperatures, the solvent molecules will have higher kinetic energy, allowing them to break the hydrogen bonds between the molecules and dissolve H₂ (g) more easily. At higher pressures, there will be more molecules of H₂ (g) in a given volume, increasing the chances of it dissolving into the solvent.
learn more about hydrogen bond Refer:brainly.com/question/21054466
#SPJ1
how to find instantaneous rate of change from a graph chemistry
Answers
The tangent line is a line that has the same slope as the curve at a single point.
The instantaneous rate of change is the slope of the tangent to the curve at a specific point, the slope of the tangent being the value of the rate of change at that time. A tangent is a straight line that touches a curve at a single point. The slope of the curve at that point is the same as the slope of the tangent at that point. The tangent line is a line that has the same slope as the curve at a single point. Therefore, by finding the slope of the tangent line, you can determine the instantaneous rate of change.The slope of a tangent to a curve at a specific point is calculated as follows;1. First, plot a graph of the data, with time on the x-axis and concentration on the y-axis.2. Pick a point on the curve.3. Draw a tangent line at that point.4. Find the slope of the tangent line.5. Repeat steps 2-4 for other points on the curve to obtain on how to find the instantaneous rate of change from a graph in chemistry.
To know more about tangent line visit:
https://brainly.com/question/23416900
#SPJ11
What is the latent heat of vaporization of boiling water?
a. 955 Btu/lb
b. 540 cal/gm
c. 2557 Kj/kg
d. 144 Btu/lb
Answers
The latent heat of vaporization of boiling water is 540 cal/gm. Option b is the correct answer.
It is the amount of heat needed to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature. The latent heat of vaporization for water is relatively high compared to other liquids because of the strong hydrogen bonding between water molecules. This means that water requires a lot of energy to break these bonds and change from a liquid to a gaseous state. The latent heat of vaporization is also responsible for the cooling effect of evaporation. When sweat evaporates from our skin, it absorbs heat from our body, which helps to cool us down. In conclusion, the latent heat of vaporization of boiling water is 540 cal/gm, which represents the amount of heat required to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature.
know more about latent heat
https://brainly.com/question/23976436
#SPJ11
3. Find the period 2 elements (atomic numbers #3-10) and the period 3 elements (#11 - 18). Do period 2 and period 3 have the same trend?
Period 2 elements are Lithium (Li), Berillium (Be), Boron (B), Carbon (C), Nitrogen ( N), Oxygen (O),
Fluorine (Fe), Neon (Ne). Period 3 elements are Sodium (Na), Magnesium (Mg), Aluminum (AI), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (CI, Argon, (Ar)
do period 2 and period 3 have the same trend ? (if they do can you explain a bit ?)
(brainly !!)
Answers
Period 2 elements are Lithium (Li), Berillium (Be), Boron (B), Carbon (C), Nitrogen ( N), Oxygen (O), Fluorine (Fe), Neon (Ne).
Period 3 elements are Sodium (Na), Magnesium (Mg), Aluminum (AI), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (CI, Argon, (Ar)
Define periodic table.
All identified chemical elements are arranged in rows (referred to as periods) and columns (referred to as groups) in the periodic table of chemical elements, also known as the periodic table, in ascending order of atomic number.
Period 3 elements have a tendency toward being hard (APART FROM Group 1), glossy, and having a metallic shine. They are also solids at room temperature and pressure (apart from mercury, which is a liquid metal), and they are good electrical conductors.
All elements in period 2 experience a decrease in atomic radius, an increase in electronegativity, and an increase in ionization energy as their atomic number rises. Only two metals (lithium and beryllium) are present in Period 2, which is fewer than any other period in terms of both quantity and proportion.
To learn more about periodic table use link below:
https://brainly.com/question/1173237
#SPJ1
Enter the electron configuration for Ne. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons
1s 2s 2p 3s 3p 3d 4s 4p 4d 4f
5s 5p 5d 5f 6s 6p 6d 7s 7p 7d
Answers
Electron configuration for Ne is \($1 s^2 2 s^2 2 p^6$\).
What is electronic configuration?The arrangement of an element's atoms and electrons among various atomic orbitals is symbolically represented by the electronic configuration of that element.
In the electronic configuration, electrons are dispersed throughout an atom's several energy levels, or "shells," including the K shell, L shell, M shell, and N shell, among others.
All electron-containing atomic subshells are placed in a particular order according to the conventional nomenclature for atomic electron configurations.
Protons, neutrons, and electrons are the three main subatomic particles found in an atom.
With a total of 10 electrons, neon is the tenth element. The initial two electrons in the electron configuration for neon will be in the 1s orbital. The following two electrons for Ne enter the 2s orbital since 1s can only hold two electrons. Six additional electrons will enter the 2p orbital.
Therefore the Ne electron configuration will be
\($1 s^2 2 s^2 2 p^6$\)
For more questions on electron configuration
https://brainly.com/question/14283892
#SPJ4
How many grams are in 2.40 moles of sodium hydroxide (NaOH)?
Answers
Answer:
96g
Explanation:
Given parameters:
Number of moles of NaOH = 2.4moles
Unknown:
Mass of NaOH = ?
Solution:
The mass of a substance given the number of moles can be found using the expression below;
Mass of NaOH = Number of moles x molar mass;
Molar mass of NaOH = 23 + 16 + 1 = 40g/mol
Now input the parameters and solve;
Mass of NaOH = 2.4 x 40 = 96g
when 0.083 moles of ammonium sulfate ((nh4)2so4) are dissolved in enough water to make 567 milliliters of solution, how many ammonium ions are present?
Answers
There are 0.83 moles of ammonium ions present in 567 mL of solution.
This can be calculated by first converting the given amount of ammonium sulfate into moles (0.083 moles) and then multiplying it by the number of ammonium ions present in each mole of ammonium sulfate (2). The resulting number is 0.83 moles of ammonium ions. This number can then be converted to milliliters using the molarity equation (M = n/V). This equation can be rearranged to solve for n (the number of moles). Plugging in the values from the given problem yields n = 0.83 moles of ammonium ions present in 567 mL of solution.
To know more about ammonium ions refer to the link brainly.com/question/11173657
#SPJ4
What will be the aproximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M? A. 96 mL B. 25 mL C. 86 mL D.1.38 x 10^2 mL
Answers
The correct option is (C). The approximate final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
To find the final volume of the solution, we can use the formula:
C1V1 = C2V2
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Plugging in the values we know, we get:
(8.25 M) (25 mL) = (2.40 M) (V2)
Solving for V2, we get:
V2 = (8.25 M x 25 mL) / 2.40 M
V2 = 86.25 mL
Therefore, the approximate final volume of the solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M is 86 mL (option C).
Learn more about final volume at: https://brainly.com/question/22012954
#SPJ11
Ayúdenme por favor
Please help me
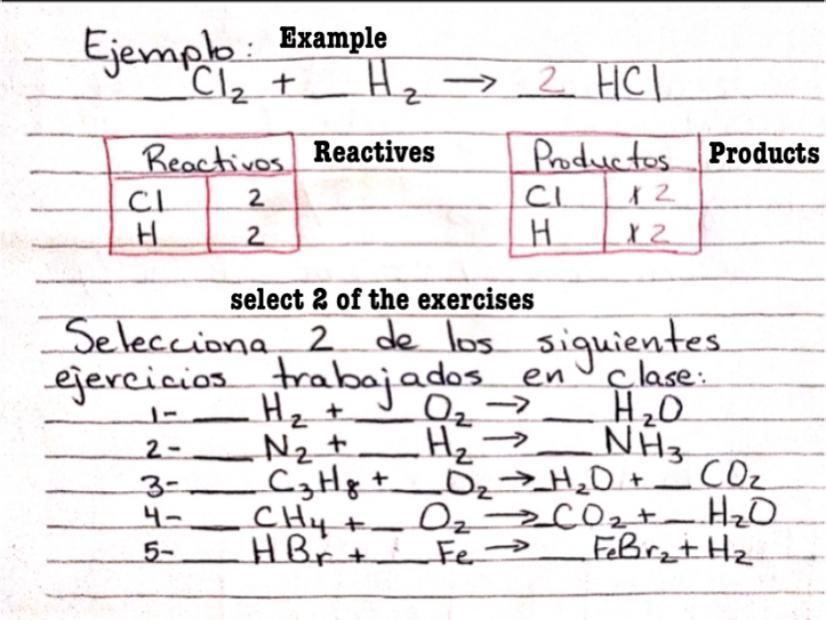
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
What are the two flasks for measuring the liquid volume?
Answers
Answer:
Liquid volume is usually measured using either a graduated cylinder or a buret.
A solution made by dissolving 25. 0 mg of insulin in 5. 00 mL of water has an osmotic pressure of 15. 5 mmHg at 25°C. Calculate the molar mass of insulin. (Assume that there is no change in volume when the insulin is added to the water and that insulin is a non-dissociating solute. )
Answers
The molar mass of insulin is approximately 0.798 g/mol, calculated using the equation for osmotic pressure and the given values of mass and volume.
To calculate the molar mass of insulin, we can use the equation for osmotic pressure:
π = (n/V)RT
where π is the osmotic pressure, n is the number of moles of solute, V is the volume of the solution in liters, R is the ideal gas constant, and T is the temperature in Kelvin.
First, convert the given values to appropriate units:
25.0 mg = 0.025 g
5.00 mL = 0.005 L
Next, rearrange the equation to solve for n (number of moles):
n = (πV) / (RT)
Substituting the given values:
n = (15.5 mmHg * 0.005 L) / ((0.0821 L·atm/(mol·K)) * 298 K)
Calculate n:
n ≈ 0.0313 mol
Finally, divide the mass of insulin (0.025 g) by the number of moles (0.0313 mol) to find the molar mass:
Molar mass = 0.025 g / 0.0313 mol
Molar mass ≈ 0.798 g/mol
So, the molar mass of insulin is approximately 0.798 g/mol.
Learn more about osmotic pressure here:
https://brainly.com/question/29823250
#SPJ11
convert 360mL of hydrochloric acid into L
Answers
In the Haber Process, ammonia is synthesized from nitrogen andhydrogen:
N2 (g) + 3H2 -----> 2NH3(g)
ΔG at 298K for this reaction is -33.3 kj/mol. the valuef ΔG at 298 K for a reaction mixture that consists of 1.9 atmN2, 1.6 atm H2 and 0.65 atm NH3 is________.
a.) -3.86 x 103
b.) -1.8
c.) -7.25 x 103
d.) -40.5
e.) -104.5
Answers
The value of ΔG at 298 K for a reaction mixture containing 1.9 atm N2, 1.6 atm H2, and 0.65 atm, the answer is (a) -3.86 × 10^3.
NH3 can be calculated using the equation:
ΔG = ΔG° + RT ln(Q)
where ΔG is the standard Gibbs free energy change, ΔG° is the standard Gibbs free energy change at standard conditions, R is the gas constant, T is the temperature in Kelvin, and Q is the reaction quotient.
In this case, we are given ΔG° as -33.3 kJ/mol. To calculate Q, we need to use the partial pressures of the gases in the reaction mixture. The reaction stoichiometry tells us that the ratio of the partial pressures of N2, H2, and NH3 is 1:3:2. Therefore, we can write:
Q = (P(NH3))^2 / (P(N2) * P(H2)^3)
Plugging in the given values of P(N2) = 1.9 atm, P(H2) = 1.6 atm, and P(NH3) = 0.65 atm, we can calculate Q. Then, using the value of R = 8.314 J/(mol·K) and the temperature T = 298 K, we can substitute these values into the equation and solve for ΔG.
The calculated value of ΔG at 298 K for the given reaction mixture is approximately -3.86 × 10^3 J/mol. This value is equivalent to -3.86 kJ/mol. Therefore, the answer is (a) -3.86 × 10^3.
To learn more about Haber Process here brainly.com/question/30928282
#SPJ11
Porosity is the fraction of material occupied by empty spaces. It can be expressed as a percent or a decimal. Assume that the porosity of a sample of sand and a sample of gravel is the same: 30 percent (0.30). Determine the porosity of a mixture of the sand and gravel, assuming that the sand fills the spaces between the gravel
Answers
Assuming that the porosity of both the sand and the gravel samples is the same at 30%, the remaining space in each sample (or the solid volume fraction) would be 70%.
When the sand and gravel are mixed together, the sand will fill the spaces between the gravel. The total volume of the mixture will be the sum of the volumes of the sand and gravel.
Let's assume we have a certain volume of gravel, and we add sand until the spaces between the gravel are completely filled. The total volume of the mixture will be greater than the volume of the gravel alone, because the sand has filled in the spaces between the gravel.
The porosity of the mixture will be the fraction of the total volume that is empty space. Since the sand has filled the spaces between the gravel, the porosity of the mixture will be less than 30%.
The exact porosity of the mixture will depend on the relative proportions of sand and gravel in the mixture, as well as the shape and size of the particles. However, we can estimate the porosity of the mixture based on the assumption that the sand fills the spaces between the gravel. If we assume that the volumes of sand and gravel are equal, then the porosity of the mixture will be:
Porosity of mixture = (Volume of empty space in mixture) / (Total volume of mixture)
Total volume of mixture = Volume of sand + Volume of gravel
If the volumes of sand and gravel are equal, then the total volume of the mixture will be twice the volume of either the sand or the gravel. Therefore:
Total volume of mixture = 2 x Volume of sand (or gravel)
Volume of empty space in mixture = Volume of gravel x Porosity of gravel + Volume of sand x Porosity of sand
Since the porosity of the sand and gravel samples are assumed to be the same at 30%, we have:
Volume of empty space in mixture = Volume of gravel x 0.30 + Volume of sand x 0.30
Substituting 2 x Volume of sand for the total volume of the mixture, we get:
Porosity of mixture = (Volume of gravel x 0.30 + 2 x Volume of sand x 0.30) / (2 x Volume of sand)
Simplifying the expression, we get:
Porosity of mixture = (Volume of gravel + 0.6 x Volume of sand) / (2 x Volume of sand)
So, the porosity of the mixture depends on the relative volumes of sand and gravel. If the volumes are equal, then the porosity of the mixture will be:
Porosity of mixture = (Volume of gravel + Volume of sand x 0.6) / (2 x Volume of sand)
For example, if we have 1 liter of sand and 1 liter of gravel, then the total volume of the mixture will be 2 liters. The volume of empty space in the mixture will be:
Volume of empty space in mixture = 1 liter x 0.30 + 1 liter x 0.30 = 0.6 liters
Therefore, the porosity of the mixture will be:
Porosity of mixture = 0.6 liters / 2 liters = 0.30 or 30%
To know more about relative volumes , visit :
https://brainly.com/question/2467054
#SPJ1
Between Na and Na+ which has larger size
Answers
Answer:
Na+ is smaller than Na because, it has given away one electron because of which the electron shielding gets stronger due to more protons and less electrons. Whereas, Cl- is larger than Cl because it has gained an extra electron and so, the no.07/12/2010
Explanation:
Which sentence in the picture is true please help me with this

Answers
Answer:
C (top to bottom) or the 3rd one
Explanation:
how much water must be added to 500.0ml of a 3.00 m hcl solution to obtain a solution that is 2.00m?
Answers
250.0 ml of water must be added to 500.0 ml of a 3.00 M HCl solution to obtain a solution that is 2.00 M.
To calculate the amount of water that needs to be added to a 500.0 ml of 3.00 M HCl solution to obtain a 2.00 M solution, we can use the following formula: M₁V₁ = M₂V₂
Where: M1 = initial concentration of the HCl solution = 3.00 M
V₁ = initial volume of the HCl solution = 500.0 ml
M₂ = final concentration of the HCl solution = 2.00 M
V₂ = final volume of the HCl solution (i.e. the volume of the HCl solution after water has been added)
Solving for V₂:
M₁V₁ = M₂V₂
(3.00 M) (500.0 ml) = (2.00 M) V2
V₂ = (3.00 M x 500.0 ml) / (2.00 M)
V₂ = 750.0 ml
Therefore, the final volume of the HCl solution after water has been added is 750.0 ml. Since we started with 500.0 ml of HCl solution, we need to add:
750.0 ml - 500.0 ml
= 250.0 ml
To know more about solution here
https://brainly.com/question/7932885
#SPJ4
Chlorine and oxygen form many different compounds, including CIO, and Cl2O3. How do the names of these compounds differentiate one chlorine oxide from another?
Answers
Answer:
We can mix two compounds and create a third that bears little resemblance to its parents. For instance, by mixing two parts of hydrogen gas with one of oxygen - liquid water is formed. We should not be misled by the fact that chlorine and chlorine dioxide share a word in common. The chemistries of the two compounds are completely different.
Chlorine and chlorine dioxide are both oxidizing agents (electron receivers). However, chlorine has the capacity to take in two electrons, whereas chlorine dioxide can absorb five. This means that mole for mole, ClO2 is 2.6 times more effective than chlorine.
If equal, if not greater importance is the fact that chlorine dioxide will not react with many organic compounds, and as a result, ClO2 does not produce environmentally dangerous chlorinated organics. For example; aromatic compounds have carbon atoms arranged in rings and they may have other atoms, such as chlorine, attached to these rings, to form a chlorinated aromatic - a highly toxic compound that persists in the environment long after it is produced.
Chlorine dioxide's behaviour as an oxidizing agent is quite dissimilar. Like ozone, the predominant oxidation reaction mechanism for chlorine dioxide proceeds through a process known as free radical electrophilic (i.e. electron-attracting) abstraction rather than by oxidative substitution or addition (as in chlorinating agents such as chlorine or hypochlorite). This means that chlorinated organic compounds such as THMs and HAAs are not produced as a result of disinfection using chlorine dioxide
Hope this helps, have a nice day! :D
The way that the names of these compounds show that the Chlorine oxides are different is by making reference to the number of individual molecules in the compound.
How are the chlorine oxides differentiated?The number of chlorine and oxygen molecules in each compound are used to name the compounds which ensures that they are differentiated.
ClO₂ is called "Chlorine Dioxide" which shows that there are two Oxygen atoms.
Cl₂O₃ is called "Dichlorine Trioxide" which shows that there are two Chlorine molecules and 3 Oxygen molecules.
Find out more on Chlorine oxides at https://brainly.com/question/25937152.
If a mineral has a metallic shine and scratches, which of its properties are being measured? Choose the two correct answers.
Answers
Choose the two correct answers.
magnetism
hardness
cleavage
luster
Answer: hardness and luster
Metals have shiny surface by which they reflect light. The two properties which can be measured from the metallic shine and scratches in a metal are its luster and hardness.
What are metals?Metals are electropositive elements conducts thermally and electrically. Almost all metals shows a shining property when they reflect light and it is called metallic luster.
Hardness of a metal can be a measure how much the metals resists scratches. Very hard metals do not form powders on scratching. Less hard metals may effect by scratches.
More hard and shining metals have various applications such as in construction fields, optoelectronic devices, thermal coatings and corrosion resistant products.
Hence, option a and b, hardness and luster can be measured from the scratches and metallic shining respectively.
To know more about metals, refer the link below:
https://brainly.com/question/18153051
#SPJ2
Your question is incomplete, but most probably your full question was:
If a mineral has a metallic shine and scratches, which of its properties are being measured?
choose the two correct answers.
a. luster
b. hardness
c. magnetism
d. cleavage
PLEASE ANSWER REALLY QUICK!!
Drag the item from the item bank to its corresponding match.
Put responses in the correct input to answer the question. Select a response, navigate to the desired input and insert the response. Responses can be selected and inserted using the space bar, enter key, left mouse button or touchpad. Responses can also be moved by dragging with a mouse.
This is to observe carefully and in detail so as to identify causes, key factors, or possible results.
This is what people do when they are searching for information. People often look in more than one location.
This is a prediction of the outcome of scientific processes based on analysis.
This is the process of steps taken in completing a task, such as a lab investigation.





Answers
We can see here that:
The cloudiest month - AprilThe coldest month - JanuaryThe hottest month - MayWhen the temperature first rose above 90 degrees - February.We see here that the bar graph will be more understandable if there is: A. Title.
Another thing that can aid in understanding the bar graph is: B. add pictures.
What is bar graph?Using rectangular bars with heights or lengths proportional to the values they represent, a bar chart or bar graph displays categorical data. Both a vertical and a horizontal bar plot are possible. A column chart is another name for a vertical bar graph.
An appropriate question to guide Sarah's research will be: C. Which fixture uses the most water in our home?
To make this a real experiment, Sarah has to do the following: D. Collect and analyze data before and after the leaks are fixed.
Learn more about bar graph on https://brainly.com/question/25196929
#SPJ1
Which molecule would you expect to be least reactive in nucleophilic acyl substitution?
Answers
Carboxylate groups are the least reactive towards nucleophilic acyl substitution among carboxylic acid derivatives, followed by amides, (protonated) carboxylic acids, thioesters, esters and finally acyl phosphates, that are most reactive among biologically relevant acyl groups.
Before delving into the details of nucleophilic acyl substitutions, it is critical to understand the relative reactivity of carboxylic acid derivatives. In general, the carbonyl carbon in an acyl group is less electrophilic than the carbonyl carbon in an aldehyde or ketone. This is because the partial positive charge on the carbon in carboxylic acid derivatives is somewhat stabilized by resonance effects from the adjacent heteroatom.
To learn more about nucleophilic acyl substitution: brainly.com/question/14273781
#SPJ4
please help me with this or I may get an F in chemistry :(

Answers
Answer:
Subconscious - psychic activity just below the level of awareness
Election - the act of electing someone
Explanation:
I hope this helped enough, good luck in chemistry!
when a substance goes directly from a solid state to a gas state as dry ice
Answers
Answer:
Sublimation
Explanation:
Sublimation refers to the process by which the change of matter takes place directly from solid to liquid state. The matter from the solid-state directly changes into the gaseous state without changing into the liquid state. More energy is required in this process. This is an endothermic reaction. Dry ice is the solid carbon dioxide sublimes in the air.
One is a negative environmental consequence of surface mining?
Answers
Answer:
Surface mining (also known as "strip mining") has the potential to significantly erode soil fertility, pollute waters, deplete underground water sources, scar or altar the landscape, damage roads, houses, and other buildings, and kill wildlife.