What patterns do you notice in the table in terms of protons, electrons, and valence electrons? how might these relate to an element being a metal or nonmetal?
Answers
The patterns in the periodic table concerning protons, electrons, and valence electrons can help us understand the properties of elements, including whether they are metals or nonmetals. The position of an element in the table and the number of valence electrons it possesses are crucial factors in determining its behavior and reactivity.
Patterns in the periodic table in terms of protons, electrons, and valence electrons, and how these might relate to an element being a metal or nonmetal.
In the periodic table, you'll notice the following patterns:
1. The number of protons (also known as the atomic number) increases by one from left to right across a period and down a group. This is because each element has one more proton than the element before it.
2. The number of electrons in a neutral atom is equal to the number of protons, so the electron count also increases by one across a period and down a group.
3. Valence electrons are the outermost electrons of an atom, and they play a significant role in chemical bonding. As you move from left to right across a period, the number of valence electrons increases from 1 to 8. In contrast, when you move down a group, the number of valence electrons remains the same.
Now, let's discuss how these patterns relate to an element being a metal or nonmetal:
1. Metals are typically found on the left side of the periodic table, while nonmetals are on the right side. This is because metals generally have fewer valence electrons (1 to 3) and are more likely to lose them in a chemical reaction. Nonmetals have more valence electrons (4 to 8) and are more likely to gain or share them.
2. The number of valence electrons determines the reactivity and bonding behavior of elements. Metals with fewer valence electrons are more reactive, while nonmetals with more valence electrons are less reactive.
In conclusion, the patterns in the periodic table concerning protons, electrons, and valence electrons can help us understand the properties of elements, including whether they are metals or nonmetals. The position of an element in the table and the number of valence electrons it possesses are crucial factors in determining its behavior and reactivity.
To know more about metal and nonmetal :
https://brainly.com/question/13963963
#SPJ11
Related Questions
I Wanted to ask.. it's a weird question I know or maybe it's gonna get reported But
Why is my friend so irritating..
Answers
Answer:
if hyper its probaly because the have ADHD which is a type of drug that helps people with behavior disorders . the might have ticks wich is something that people can not control.
Explanation:
a chemist needs to prepare 2.50 l of a 0.350 m solution of potassium permanganate (kmno4 ). what mass of kmno4 does she need to make the solution?
Answers
Mass of kmno4 does she need to make the solution is 128.375 g.
Data:-Volume = 2.5 L
Concentration = 0.325 M
Potassium permanganate (Molar mass )= 39 + 55 + (16 x 4) = 158 g
Calculate the moles of KMnO₄
Formula
Molarity = moles/volumemoles = molarity x volume
moles = 0.325 x 2.5
moles = .8125
Calculate the grams of KMnO₄
158 g KMnO₄ ------------------ 1 mol
x ------------------ .8125
x = (1.95 x 158)/1
x = 128.375 g
What distinguishes mole molarity from molality?The amount of a solute in moles to all the liters of a solution is known as its molarity. The solvent and solute are both present in the solution. The ratio of the moles of a solute to the kilograms of a solvent is known as molality, on the other hand.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ4
Which statement below is not supported by the
kinetic-molecular theory?
The total pressure of a mixture of gases is equal
to the sum of the pressures of all of the gases in
the mixture.
b. The volume of the gas particles is small
compared with the volume of empty space
between the particles.
c. Gas particles are in constant, random motion,
and collisions between them are elastic.
d. At a given temperature, all gases have the same
average kinetic energy.
Answers
The statement below which is not supported by the kinetic-molecular theory is that the total pressure of a mixture of gases is equal to the sum of the pressures of all of the gases in the mixture which is therefor denoted as option A.
What is Kinetic-molecular theory?This states that gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant, random motion.
The total pressure by a mixture of gases is equal to the sum of the partial pressures of each of the constituent gases and at a given temperature, all gases have the same average kinetic energy thereby making option A the correct choice.
Read more about Kinetic-molecular theory here https://brainly.com/question/134712#:~:text=Expert%2DVerified%20Answer&text=the%20kinetic%20molecular%20theory%20explains,and%20forces%20between%20the%20particles.
#SPJ1
If one person reacts to something in a certain way, that means ALL people will react to the
same situation in the same way (Johnny gets stung by a bee, now he kills every bee he sees).
O TRUE
O FALSE
Answers
Hi there,
My answer would be False.
Please mark
Thanks
The statement is false that if one person reacts to something in a certain way, that means ALL people will react to the same situation in the same way (Johnny gets stung by a bee, now he kills every bee he sees).
What makes this statement false?According to this question, if one person reacts to something in a certain way, that means ALL people will react to the same situation in the same way.
An example then states that Johnny gets stung by a bee and begins to kill every bee he sees.
This statement is false because when Johnny gets stung by a bee, everyone should react to the situation the same way Johny Johnny did.
Learn more about false statements at: https://brainly.com/question/24701431
how is soda made?
this includes sprite, coca cola, dr pepper, exd.
Answers
Answer:
Soda all start with watered down corn syrup. To get the carbonation companies have different ways of doing this all of which are very different. The last step is to add artificial and natural flavors like lemon and lime, this is also the step in which sugar and preservatives are added.
Explanation:
I hope this answer your question, srry I couldn't go into detail.
6. In which of the following will the bulb glow?
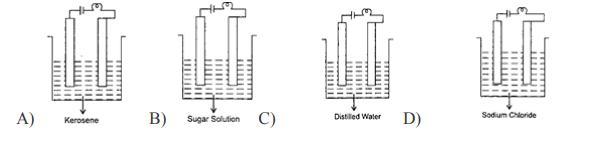
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
las diferencias fundamentales del entramado urbano antes y despues de 1750
Answers
Answer:?
?Explanation:
Question 57
Marks: 1
The EPA stream quality indicator for dissolved oxygen in stream water is
Choose one answer.
a. 3 mg per liter
b. 4 mg per liter
c. 5 mg per liter
d. 6 mg per liter
Answers
The EPA stream quality indicator for dissolved oxygen in stream water is:c. 5 mg per liter
Dissolved oxygen (DO) is an important indicator of the health of a water body. The EPA (Environmental Protection Agency) has set guidelines for the minimum dissolved oxygen levels to support a healthy aquatic ecosystem. For streams, the recommended minimum level of dissolved oxygen is 5 mg per liter. This level ensures that the water can support a diverse range of aquatic life, including fish, invertebrates, and other organisms.
To maintain good water quality, it's essential to regularly monitor dissolved oxygen levels using various sampling methods and equipment. If dissolved oxygen levels drop below the recommended threshold, it can indicate problems such as pollution or excessive nutrient loading, which can lead to eutrophication and negatively impact the ecosystem.
Learn more about Dissolved oxygen Refer: https://brainly.com/question/29027453
#SPJ11
Which state of matter represented by the particles

Answers
Answer:
A solid
the internolecular forces are packed and leave no space behind
What is called exothermic
Answers
An exothermic process is one that gives off heat.
Explanation:
This heat is transferred to the surroundings. An endothermic process is one in which heat has to be supplied to the system from the surroundings.
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
can you pair electrons in degenerate orbitals before filling each orbital halfway
Answers
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
why is it necessary to balance chemical equations
Answers
Answer:
to satisfy the law of conservation of mass
Explanation:
have great day
Answer:
It is necessary for chemical equations to be balanced because of the law of conservation of mass (the mass of the products = the mass of the reactants).
Chemical equations follow/represent this law
what volume (l) of nh3 gas at stp is produced by the complete reaction of 7.5 g of h2o according to the following reaction?
Mg3N2(s)+6H2O(I) arrow 3Mg(Oh)2 +2NH3
Answers
The given balanced equation is:Mg3N2(s) + 6H2O(l) → 3Mg(OH)2(s) + 2NH3(g)The stoichiometric ratio of the number of moles of H2O and NH3 is 6:2 or 3:1. Therefore, 7.5 g of H2O produces (2/3) × 7.5 g of NH3=5 g of NH3.
Now, we need to calculate the volume (L) of NH3 gas at STP is produced by the complete reaction of 7.5 g of H2O.According to ideal gas lawPV = nRTwhere, P = pressureV = volumeT = temperaturen = number of moles of gasR = gas constantIn case of STP, P = 1 atm, T = 273 K, and R = 0.082 L atm K−1 mol−1Now, n = mass/molar mass=5 g / 17 g mol¯¹ (molar mass of NH3)= 0.2941 molSo, PV = nRTV = (nRT)/PV = (0.2941 mol × 0.082 L atm K−1 mol−1 × 273 K) / 1 atm= 6.35 LAns: The volume (L) of NH3 gas at STP produced by the complete reaction of 7.5 g of H2O is 6.35 L.
To know more about reaction, visit ;
https://brainly.com/question/11231920
#SPJ11
Please help witht 4 and 5

Answers
Answer:
#4: 0.89 mol/L #5: 0.36 mol
Explanation:
#4: Morality is Moles/ Liters
So take 3.2 mols ÷ 3.6 L = 0.8888... since there are 2 sig figs in the question the answer would round to 0.89 mol/L
#5: to find the number of moles needed, you take the mol/L and multiply that by the volume.
0.89 mol/L ×0.400L = 0.356 mols. the liters cancel out. Since 0.89 has the least number of sig figs your final answer should have 2. which it 0.36 mols.
I hope this helps, good luck!
a calorimeter has a heat capacity of 1265 j/o c. a reaction causes the temperature of the calorimeter to change from 22.34o c to 25.12o c. how many joules of heat were released in this process?
Answers
The amount of heat energy (q) released in the reaction can be calculated using the following formula:q = CΔTWhere C is the heat capacity of the calorimeter and ΔT is the change in temperature. Substituting the values into the formula, we get:q = 1265 J/°C × 2.78°Cq = 3520.7 Therefore, 3520.7 joules of heat were released in the reaction.
A calorimeter is an instrument used to measure the amount of heat energy transferred to or from a substance in a chemical reaction. It is designed to prevent heat exchange between the system and the surroundings.The heat capacity of the calorimeter is the amount of heat energy required to raise its temperature by one degree Celsius. In this case, the heat capacity of the calorimeter is 1265 J/°C.The temperature of the calorimeter was initially 22.34°C and it changed to 25.12°C due to a chemical reaction. The change in temperature (ΔT) can be calculated by subtracting the initial temperature from the final temperature.ΔT = T₂ - T₁ = 25.12°C - 22.34°C = 2.78°CSince the calorimeter is designed to prevent heat exchange with the surroundings, the heat energy released in the reaction is absorbed by the calorimeter. The amount of heat energy (q) released in the reaction can be calculated using the following formula:q = CΔTWhere C is the heat capacity of the calorimeter and ΔT is the change in temperature.Substituting the values into the formula, we get:q = 1265 J/°C × 2.78°Cq = 3520.7 JTherefore, 3520.7 joules of heat were released in the reaction.
To Know more about heat energy visit:
brainly.com/question/29210982
#SPJ11
Which sings shake the room A pop smoke and Quavo B Roddy Ricch C YoungBoy never Broke Again D lil yatchy
Answers
Answer:
YoungBoy Never Broke Again. Explanation: He is the goat
Hi can someone please help me!

Answers
Answer:
a) 2
b) 2
c) 5
d) 5
e) 5
Explanation:
a) There is 1 Ag atom and 1 Cl atom. When there's no subscript number next to an element, it means there is only one.
b) There is 1 Ca atom and 1 O atom.
c) There are 3 Mg atoms (there's a subscript 3 next to Mg) and 2 N atoms.
d) There are 2 Al atoms and 3 O atoms.
e) There are 2 Sc atoms and 3 S atoms.
Research on ‘where a person’s carbon emissions come from inside a home’ and represent the data in the form of a pie chart. Pls answer with pie chart I WILL MARK U AS BRAINSLIEST IF YOU GIVE RIGHT ANSWER
Answers
Answer:
Here's what I get from one source.
Explanation:
\(\begin{array}{lc}\textbf{Source} &\textbf{Contribution \%} \\\text{Space heating }& 45\\\text{Water heating} &18\\\text{Space cooling} & 9 \\\text{Computers/Electronics} & 6 \\\text{Lighting} & 6 \\\text{Cooking} & 4 \\\text{Refrigeration} & 4 \\\text{Wet cleaning} & 3 \\\text{Other} & 5 \\\end{array}\)
The pie chart is in the Figure below.

Which kind of molecular shape will this molecule have? H-S-H
Answers
Answer:
the molecule is formed by covalent bond , and each hydrogen atom forms a single bond with sulphur atom.
What happens to water once it moves from earths surface to the atmosphere
Answers
Answer:
Evaporation
Explanation:
Water at the surface of the ocean rivers and lakes can become water vapor and move into the atmosphere with a little added energy from the Sun through a process called evaporation. Snow and ice can also become water vapor through a process called sublimation. See also why do variations in generation time exist
post lab questions 1. is the flame color a test for the metal or for the chloride in each compound? explain your answer? 2. why do different metals have different characteristic flame test colors? 3. most salts contain a metal and a non-metal. look at the compounds we tested and determine whether it is the metal or the non-metal that is responsible for the color produced in the flame test for that salt. how can you be sure your answer is correct? 4. why do the chemicals have to be heated in the flame before the colored light is emitted? 5. could flame tests be useful in determining identities of metals in a mixture of two or more salts? if so, what problems might arise? if not, why not? explain your answer. 6. construct the line spectra for each metal used in the experiment. (can use pictures from the net with cut and paste. spectra must be in full color and only individual lines must be present. 7. using the one brightest (color) wavelength for each cation, select a wavelength value from the chart above
Answers
Different metals create particular colored flames during in the flame test since each metal has a different arrangement of electrons, which produces specific colors of light when heated. Since the visible flame color is only of the metal alone, the metal may be recognized.
Why does each metal's typical flame test color differ?The precise sizes of the potential energy jumps differ from metal to metal.As a result, the flame color of each metal will differ due to its unique spectral line pattern.The movement of a electrons inside the metal ions contained in the compounds results in the hues of the flame.
Why do various elements produce flames of varying hues?Because the energy levels of the electrons travelling around the nucleus fluctuate depending on the element, different compounds react to fire to produce distinct colored flames.
To know more about post lab questions visit:
https://brainly.com/question/27952101
#SPJ4
Why is the condensation of water vapor considered to be a process which hads up the air? a. Water yapar must nbsorb energy in order to condense. b. Air cain hold thore water in the liquld phase that the vapor phase. c. Energy is released by water vapor as it condenses. d. Liquid water has a lower specific heat than water vapor. QUESTION 60 a. 42% b. 2+5% c 90% d. 3376
Answers
The correct answer to the first part of your question is option (c): Energy is released by water vapor as it condenses.
When water vapor condenses into liquid water, it undergoes a phase change from a gaseous state to a liquid state. During this phase change, energy is released in the form of latent heat. This release of energy occurs because the water molecules in the vapor phase are more energetic and have higher kinetic energy compared to the water molecules in the liquid phase.
To know more about latent heat
brainly.com/question/23976436
#SPJ11
Nitrogen gas and hydrogen gas react to produce ammonia according to the following equation.
N2 + 3H2 → 2NH3
Which ratio of components is correct?
A For every 2 moles of nitrogen gas, the reaction requires 3 moles of hydrogen gas.
B For every 3 moles of hydrogen gas, the reaction produces 2 moles of ammonia.
C For every mole of hydrogen gas, the reaction produces 2 moles of ammonia.
D For every mole of nitrogen gas, the reaction produces 1 mole of ammonia.
Answers
Answer:
The answer will be "B"
Explanation:
It is B, because as we can see in the problem, it shows that there are three hydrogen gas, which is H₂. So, it shows that the ratio of Hydrogen gas to Ammonia is 3:2. In the answer, it says for every 3 mol of H₂ 2 moles of ammonia is produce. Therefore, B is correct.
Have a good day, any questions let me know. If you want, you can mark as brainliest if you like the answer!
an open flask sitting in a lab refrigerator looks empty, but it is actually filled with a mixture of gases called air. if the flask volume is 2.50 l, 2.50 l, and the air is at standard temperature and pressure, how many gaseous molecules does the flask contain?
Answers
At standard temperature and pressure (STP), a 2.50 L flask will contain 2.50 L x (1 mole/22.4 L) x (6.022 x 10^23 molecules/mole) = 6.50 x 10^25 molecules.
At standard temperature and pressure (STP), the ideal gas law states that the pressure of a gas is directly proportional to its absolute temperature and inversely proportional to its volume. This means that, for a given temperature, the number of gas molecules in a flask is inversely proportional to the volume of the flask.
For example, if we have a flask with a volume of 2.50 L, then the number of gas molecules it contains can be calculated by dividing the total number of moles by the volume of the flask. The total number of moles can be calculated by multiplying the number of moles per liter (1 mole/22.4 liters at STP) by the volume of the flask (2.50 L). Multiplying the number of moles by Avogadro's constant (6.022 x 10^23 molecules/mole) yields the total number of molecules in the flask. Thus, a 2.50 L flask at STP will contain 2.50 L x (1 mole/22.4 L) x (6.022 x 10^23 molecules/mole) = 6.50 x 10^25 molecules.
Learn more about standard temperature and pressure (STP):
https://brainly.com/question/7581651
#SPJ4
How does electron pair repulsion determine the molecular shape/molecule geometry?.
Answers
The repulsion of the electron pair surrounding the core atom has a significant impact on the geometry or shape of the electron.
How does the molecule shape indicate or are determined by electron pair repulsion?The tendency of the electron pairs in an atom to repel one another when they are present is known as electron pair repulsion.
The more repulsion there is between the electron pairs, the more the electrons want to organize themselves to lessen it.
Here, the issue of how electron pair repulsion affects molecule geometry is raised.
The fundamental explanation is that the molecule will change its structure to keep the repelling electron pair apart.
The electron pair repulsion also plays a significant role in determining molecular geometry, since molecules prefer to modify their form and geometry in response to the electron pair.
To know more about molecule visit:
brainly.com/question/19922822
#SPJ4
I have a 100 g block of iron with a density of 7.874 g/cm3. How much bare
cylindrical wire can be made from this block of iron with a diameter of 1 cm
(assuming no losses in the extruding process) ? Show your work for full credit.
Answers
Answer:
1,270 cm
Explanation:
100 g (cm^3/7.874 g)(m/cm^3)(100cm/m)
= 1270 cm
Propose an explanation for why the element carbon, rather than sodium, is important in forming natural polymers?
I need this ASAP
Answers
what energy change occur when hydrogen burns
Answers
Answer:
See below.
Explanation:
When hydrogen burns, it would release energy. Not absorb energy!
In a flame of pure hydrogen gas, burning in air, the hydrogen (H2) reacts with oxygen (O2) to form water (H2O) and releases energy. If carried out in atmospheric air instead of pure oxygen, as is usually the case, hydrogen combustion may yield small amounts of nitrogen oxides, along with the water vapor.