What part of the atom is responsible for the behavior of the alpha particles?
Answers
Answer:
nucleus
Alpha particles are subatomic fragments consisting of two neutrons and two protons. Alpha radiation occurs when the nucleus of an atom becomes unstable (the ratio of neutrons to protons is too low) and alpha particles are emitted to restore balance.
Explanation:
Related Questions
3. For a demonstration, a teacher reacts 5.38 g of potassium iodide, KI, and 10.5 g lead(II) nitrate,
Pb(NO3)2.. The reaction proceeds as shown in the chemical equation below.
Pb(NO3)2(aq) + 2KI(aq) → 2KNO3(aq) + Pbl₂(s)
After filtering and drying the precipitate, the teacher determines that 7.02 g of lead(II) iodide, PbI2, have
been produced.
a) What is the theoretical yield of Pbl2(s)?
b) What is the percentage yield of Pbl₂(s)?
c) What is one possible explanation for the answer to part b)?
Answers
The percent yield of the solution is obtained as 94%.
Percent yield of a reactionWe know that the reaction equation for the problem that we have here can be written as;
Pb(NO3)2(aq) + 2KI(aq) → 2KNO3(aq) + Pbl₂(s)
Number of moles of Pb(NO3)2 = 10.5 g /331 g/mol
= 0.0317 moles
Number of moles of KI = 5.38 g/166 g/mol
= 0.0324 moles
If 1 mole of Pb(NO3)2 reacts with 2 moles of KI
0.0317 moles of Pb(NO3)2 reacts with 0.0317 * 2/1
= 0.0634
Thus KI is the limiting reactant.
Then;
2 moles of KI produces 1 mole of PbI2
0.0324 moles of KI will produce 0.0324 moles * 1 mole/2 moles
= 0.0162 moles of PbI2
Mass of PbI2 produced is;
0.0162 moles * 461 g/mol
= 7.47 g
Thus percent yield = 7.02 g / 7.47 g * 100/1
= 94%
Learn more about percent yield:https://brainly.com/question/31603690
#SPJ1
14. The atoms of element X contains nineteen electrons. With which of the following elements will the chemistry of Z be similar? a Aluminum b) Bromine c) Lithium d) Magnesium
Answers
First of all, Z is unknown. I hope it is a mistake.
Now, it is given that the element X has nineteen electrons. This proves that X is actually Potassium.
As per the periodic table, both Potassium and Lithium belongs to group 1 as their valency is 1 because of the presence of only one electron in the outermost shell of electrons i.e., they lose an electron during a chemical reaction to form a stable compound. Furthermore, both are metallic.
Magnesium belongs to group 2 and hence its valency is two, which is different from potassium though it is metallic. Similiarly, bromine belongs to group 17 and gains one electron during a reaction in contrast to potassium.
( No internal links available for reference. For clarification, check the periodic table).
In one model of the hydrogen atom, an electron orbits a proton in a circle of radius 5.28×10-11 m with a speed of 2.18×106 m/s. What is the acceleration of the electron in this model?
Answers
To find the acceleration of the electron, we can use the formula for centripetal acceleration, which is given by a = v^2/r, where v is the speed of the electron and r is the radius of the circle. The acceleration of the electron in this model is 9.00 x 10^22 m/s^2.
Plugging in the values given in the problem, we get:
a = (2.18×10^6 m/s)^2 / (5.28×10^-11 m)
a = 9.03×10^22 m/s^2
Therefore, the acceleration of the electron in this model is 9.03×10^22 m/s^2.
In the given model of the hydrogen atom, an electron orbits a proton in a circle of radius 5.28 x 10^-11 m and has a speed of 2.18 x 10^6 m/s. To find the acceleration of the electron, we can use the centripetal acceleration formula:
Centripetal acceleration (a) = (v^2) / r
where:
v = speed of the electron (2.18 x 10^6 m/s)
r = radius of the orbit (5.28 x 10^-11 m)
Now, let's calculate the acceleration:
a = (2.18 x 10^6 m/s)^2 / (5.28 x 10^-11 m)
a = 4.7524 x 10^12 m^2/s^2 / 5.28 x 10^-11 m
a = 9.00 x 10^22 m/s^2
So, the acceleration of the electron in this model is 9.00 x 10^22 m/s^2.
Learn more about proton at: brainly.com/question/1252435
#SPJ11
PLZZZZZ HELPP How do you predict the bond length and energy would change if hydrogen formed a bond with Astatine (At)?
Answers
The bond length and energy of the bond formed between hydrogen (H) and astatine (At) would change if the bond order increases or decreases or when its potential energy is at the lowest.
What is bond length?Bond length is also referred to as bond distance and it can be defined as an equilibrium distance between the nuclei of two (2) covalently bonded atoms in a molecule.
What is bond energy?Bond energy can be defined as the amount of chemical energy that is required to break one mole of a given chemical bond.
In Chemistry, the bond length and energy of a molecule is typically determined by the following:
The bond order or number of bonded electrons.The potential energy possessed by the molecule.In conclusion, you can predict that the bond length and energy of the bond formed between hydrogen (H) and astatine (At) would change if the bond order increases or decreases or when its potential energy is at the lowest.
Read more on bond energy here: brainly.com/question/5650115
what is the reducing agent in the redox reaction represented by the following cell notation? mg (s) | mg 2 (aq) || li (aq) | li (s) what is the reducing agent in the redox reaction represented by the following cell notation? (s) | (aq) || (aq) | (s) mg 2 (aq) pt mg (s) li (aq) li (s)
Answers
The reducing agent in the redox reaction represented by the following cell notation is magnesium (Mg) in the cell notation: Mg(s) | Mg2+(aq) || Li+(aq) | Li(s).
In the given cell notation, the species on the left side of the cell notation (Mg) is undergoing oxidation, which means it is losing electrons and being oxidized. The species on the right side (Li) is undergoing reduction, which means it is gaining electrons and being reduced.
In a redox reaction, the reducing agent is the species that donates electrons to another species, causing it to be reduced. In this case, magnesium (Mg) is losing electrons and being oxidized, so it is the reducing agent in the reaction.
It's important to note that the reducing agent is always the species that is being oxidized in a redox reaction, as oxidation and reduction occur simultaneously.
To learn more about redox reaction click here
brainly.com/question/28300253
#SPJ11
Is rubber band stretching physical or chemical
Answers
Answer:
physical
Explanation:
this is a physical action to create a change. does not involve chemicals or elements
Answer:
\(\boxed {\boxed {\sf Physical \ change}}\)
Explanation:
A chemical change, as the name suggests, must result from a change in the chemical makeup and new substances are produced. A very common example is metal rusting.
A physical change can be observed without a change in chemical composition. No new substances are produces. Ripping paper and an ice cube melting are physical changes.
A rubber band stretching is a physical change. No new substances are made and the chemical makeup remains the same.
FILL IN THE BLANK. in a mixture of 5 ml water, 10 ml alcohol, and 50 ml acetone the solvent(s) is(are) ________.
Answers
The solvent here is acetone.
When we mix two miscible liquids, the one with the largest amount will act as the solvent. Here water, alcohol, acetone are miscible liquids. That is all three are soluble in each other. In such cases the one with the maximum quantity will be the solvent.
So, since acetone is in largest quantity, that is, 50ml, it will act as the solvent.
For further information about solvents, please refer
https://brainly.com/question/29641339
How does the activity series of metals reflect the stability of metals?
A. The metals lowest on the list are the least stable.
B. The metals highest on the list are the most stable.
C. The metals lowest on the list are the most reactive.
D. The metals highest on the list are the least stable.
Answers
The activity series of metals reflect the stability of metals are "the metals highest on the list are the least stable."
What is activity series?The organization of metals in a declining sequence of respective reactivities is referred to as the "activity series," also described as the "reactivity series" of metals.
What is metals?Materials having the qualities of being bright, hard, fusible, malleable, ductile, etc. were known as metals.
The activity series of metals reflect the stability of metals are "the metals highest on the list are the least stable."
To know more about activity series and metals
https://brainly.com/question/14129976
#SPJ2
NEED IT ASAP
1) Plants need water for______in order to make food. water is needed more for _____, to maintain body temperature and transport nutrients.
Answers
Answer:
Hi Lovelys!!
Answer down below!!
Explanation:
Answer: Plants need water for photosynthesis in order to make food. Water is needed more for cooling, to maintain body temperature and transport nutrients.
Reason: Plants need water for photosynthesis. Water also helps move nutrients from the soil into the plant. Too little water can cause a plant to wilt or droop. Too much water can cause a plant's roots to rot. Plants are about 80-95% water and need water for multiple reasons as they grow including for photosynthesis, for cooling, and to transport minerals and nutrients from the soil and into the plant.
Hope this helps!! =3
Have a wonderful day, evening, or night!!
how does understanding the structure and function of the human brain help with diagnosis of disease
Answers
Answer:
one sec
Explanation:
Help me out here please???

Answers
Answer:
A
Explanation:
Answer:
B
Explanation:
In a chemical reaction, the number of each element should be equal on both the reactant and product side.
(ii) When shale gas is burned, the hydrogen sulfide reacts with oxygen.
2H2S + 3022SO2 + 2H2O
Explain how the combustion of shale gas can lead to the formation of acid rain.
Answers
Answer:
See explanation
Explanation:
Now , we have the equation of the reaction as;
2H2S(g) + 302(g)------->2SO2(g) + 2H2O(g)
This equation shows that SO2 gas is produced in the process. Let us recall that this same SO2 gas is the anhydride of H2SO4. This means that it can dissolve in water to form H2SO4
So, when SO2 dissolve in rain droplets, then H2SO4 is formed thereby lowering the pH of rain water. This is acid rain.
Based on the ion structure, which of the following elements would form a bond with calcium in a one to one ratio and why?
O A. One selenium ion would bond to one calcium ion because the combination of their charges would result in a full octet.
OB. One bromine ion would bond to one calcium ion because they are both located on period 4.
OC. One selenium ion would bond to one calcium ion because they are both located on period 4.
OD. One bromine ion would bond to one calcium ion because the combination of their charges would result in a full octet.

Answers
Answer:
a
Explanation:
Based on the ion structure, one selenium would form a bond with calcium in a one to one ratio because the combination of their charges would result in a full octet and the correct option is option A.
What is ionic bond?
Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Electron transfer produces negative ions called anions and positive ions called cations. These ions attract each other.
Ions are formed by atoms to gain the stability by achieving the next nearest noble gas configuration.
In Calcium,
Number of electrons = 20
Electronic configuration of Ca = 2,8,8,2
In Selenium,
Number of electrons = 34
Electronic configuration = 2,8,8,16
So, Ca has 2 electrons in the outermost shell that can be donated to form the ionic bond and Selenium needs 2 electrons to gain the nearest noble gas configuration.
Thus, one Calcium donates it two electrons forming Ca²⁺ and one Selenium gains 2 electrons forming Se²⁻
Therefore, based on the ion structure, one selenium would form a bond with calcium in a one to one ratio because the combination of their charges would result in a full octet and the correct option is option A.
Learn more about Ionic bonds, here:
https://brainly.com/question/29772028
#SPJ5
Use the atomic model below to answer the following questions: 1 pt for each blank
How many protons? How many neutrons? What is the name of this atom?
its a k12 test question TwT
Answers
Answer:
Explanation:
ALRIGHT EPIC GAMER IM GONNA INTRODUCE YOU TO THE AWESOME WORLD OF THE PERIODIC TABLE
look at this
6
C
Carbon
12.011
ok so this is what one block on the periodic table looks like
the protons and electrons are going to be the smaller number
so the protons and electrons for carbon are 6
but your question does not ask for electrons so its fine.
How many protons? LOOK AT THE SMALLER NUMBER IN ELEMENT BLOCK ON PERIODIC TABLE.
How many neutrons? Take the bigger number and subtract the smaller number from it. so it would be for carbon: 12.011 - 6 = 12 neutrons.
to find the name of the atom, just count the number of electrons and then look for the element with the same number to find the name
Calculate the relative formula mass of strontium nitrate, Sr(NO3)2.
(relative atomic masses: N = 14, O = 16, Sr = 88)
Answers
Answer:
its 210
Explanation:
Just add all atomic mass used in the formula together: 2*(14+16*3)+88= 2*62+88=124+88=210
Hope this was helpful
The relative formula mass of strontium nitrate Sr(NO₃)₂ is 210
The relative atomic masses of N, O and Sr are 14,16 and 88 respectively.
In calculating the relative atomic mass of an element with isotopes, the relative mass and proportion of each is taken into account. Adding the atomic masses together gives the relative formula mass of a compound
So, relative atomic mass of Sr(NO₃)₂ is calculated as
88+ 2(14+16×3) = 210
The atomic mass constant (symbol: mu) is defined as being 1/12 th of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless; hence the value is said to be relative atomic mass.
To know more about strontium nitrate here
https://brainly.com/question/26177156
#SPJ2
From the list below,choose which groups are part of the periodic table?
Answers
From the list provided, the following groups are part of the periodic table are Metals, Nonmetals , Semimetals and Conductors .
Metals: Metals are a group of elements that are typically solid, shiny, malleable, and good conductors of heat and electricity. They are located on the left-hand side and middle of the periodic table.
Nonmetals: Nonmetals are elements that have properties opposite to those of metals. They are generally poor conductors of heat and electricity and can be found on the right-hand side of the periodic table.
Semimetals: Semimetals, also known as metalloids, are elements that have properties intermediate between metals and nonmetals. They exhibit characteristics of both groups and are located along the "staircase" line on the periodic table.
Conductors: Conductors are materials that allow the flow of electricity or heat. In the context of the periodic table, certain metals and metalloids are good conductors of electricity.
Therefore, the groups that are part of the periodic table are metals, nonmetals, semimetals, and conductors. The other groups mentioned, such as acids, flammable gases, and ores, are not specific groups found on the periodic table but may be related to certain elements or compounds present in the table.
Know more about periodic table here:
https://brainly.com/question/15987580
#SPJ8
The complete question is :
From the list below, choose which groups are part of the periodic table.
metals
acids
flammable gases
nonmetals
semimetals
ores
conductors
A radioactive element has a half life of 10 days how many days would it take for 1000 grams of it to decay to 125 grams
Answers
A radioactive element has a half life of 10 days then there are 7 days would it take for 1000 grams of it to decay to 125 grams .
Calculation ,
radioactive element has a half life = 10 days ( given )
Kt = 0.069
K = 0.069 / t
K = 0.069 / 10 days = 0.0069
Kt = ㏑a/a-x
where ,
a = concentration of radioactive element at time 0 = 1000 grams
a-x = concentration of radioactive element at time t = 125 grams
Putting the value of a , a- x and K in equation ( i ) we get ,
0.0069 × t = ㏑1000 grams/125 grams = ㏑8 = 2.07
t= 2.07 / 0.0069 = 7 .05 days or 7 days
to learn about radioactive element
https://brainly.com/question/23759636
#SPJ1
I need help me I need helps
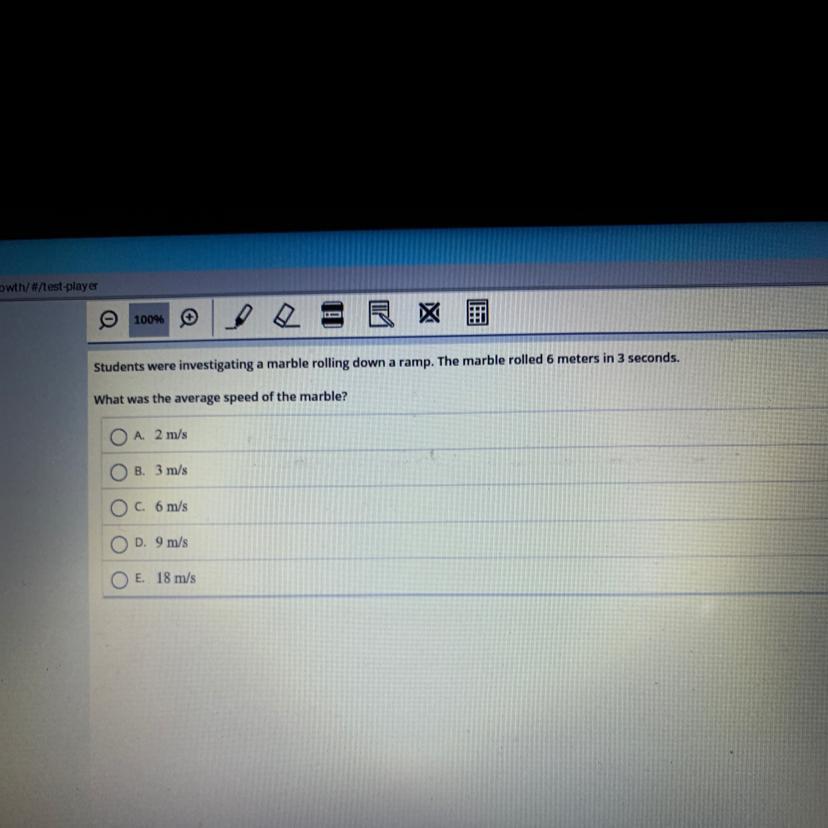
Answers
Answer:
a
Explanation:
Answer:
2 A
Explanation:
Examine the reaction. NH4OH(aq) →H2O(l) + NH3(g)
What coefficients will balance the equation?
A) 1, 1, 1
B) 3, 3, 4
C) 2, 1, 2
D) 1, 2, 2
Answers
Answer: A. 1,1,1
Explanation:
The coefficients that will balance the equation; NH4OH(aq) →H2O(l) + NH3(g), is 1, 1, 1, because it proves the total number of atoms of each element on the LHS and RHS of the equation are equal, hence balanced.
LHS RHS
N = 1 1
H = 5 5
O = 1 1
how would the molarity be affected if the base buret contained small amounts of water in it before adding the basse solusion
Answers
If the base buret contains small amounts of water before adding the base solution, the molarity of the base will be lower due to dilution, potentially leading to inaccuracies in experimental results.
Molarity (M) is defined as the number of moles of solute per liter of solution. If a base buret contains water before adding the base solution, this will increase the total volume of the resulting mixture, thereby affecting the molarity of the base. Specifically, the molarity of the base solution will be lower than initially intended.
This decrease in molarity occurs because of dilution, which is the process of reducing the concentration of a solute in a solution by adding more solvent (in this case, water). When the buret contains water before adding the base solution, the added water acts as a solvent, diluting the base and lowering its concentration.
The impact on molarity may cause inaccuracies in experimental results that rely on the precise measurement of the base solution's concentration. For instance, in a titration experiment, an incorrect molarity might lead to errors in calculating the concentration of an unknown solution. To prevent such issues, it is crucial to ensure that the base buret is properly cleaned and dried before use to avoid any unwanted dilution.
Know more about titration here:
https://brainly.com/question/31271061
#SPJ11
What is the limiting reactant if 12 moles of p4 react with 15 moles of o2?.
Answers
Answer:
see explanation
Explanation:
To determine limiting reactant divide mole quantities of reactants by the respective coefficient in the balanced equation. The smaller value is the limiting reactant.
P₄ + 5O₂ => 2P₂O₅
12/1 = 12 15/5 = 3
O₂ is the limiting reactant. P₄ will be in excess when rxn stops.
How many L in 1.98m solution using 4.2mol
Answers
The volume needed to make 1.98 M of the solution is 2.12L
Molarity of a given solution is defined as the total number of moles of solute per litre of solution. One molar is the molarity of a solution where one gram of solute is dissolved in a litre of solution. As we know, in a solution, the solvent and solute blend to form a solution, hence, the total volume of the solution is taken.
Given,
Molarity = 1.98m
Moles = 4.2 mol
Molarity = number of moles of solute / volume of solution in L
Volume = moles / molarity
= 4.2 / 1.98
= 2.12 L
Learn more about Molarity, here:
https://brainly.com/question/8732513
#SPJ1
In science, a summary of observed behavior is referred to as a(n)
Answers
Answer:
Law
Explanation:
A law is a summary of observed (measurable) behavior, whereas a theory is an explanation of behavior. A law tells what happens; a theory (model) is our attempt to explain why it happens.
In order for the part to deform more naturally, ___________ elements should be used.
Answers
In order for the part to deform more naturally, small elements should be used.
What is SOLIDWORKS SimulationXpress?SOLIDWORKS SimulationXpress users have access to a simple first-pass stress analysis tool. SimulationXpress can assist you in reducing costs and accelerating time-to-market by evaluating your designs digitally as opposed to through expensive and time-consuming field trials.Stress analysis is carried out by SimulationXpress using the same simulation technology as SOLIDWORKS Simulation. Fixtures, loads, and material qualities all influence how accurately the findings are produced. How does SimulationXpress works?Using the part file you provided, SimulationXpress splits the entire component into straightforward triangular volumes, also known as elements, that are joined at common nodes. The sum of the elements known as mesh is used by the software to forecast how the part would behave.By changing the slider, SimulationXpress allows you to modify the element sizes in the mesh.Smaller elements typically allow for a more natural deformation, whereas bigger ones can overly stiffen the part.To learn more about SOLIDWORKS SimulationXpress visit:
https://brainly.com/question/11837039
#SPJ1
A coin collector has a bag of 50 pennies. What is weighted average of a penny in the sample according to the data?

Answers
The weighted average of a penny is = 67.9g
Calculation of average weightThe sample of penny for pre 1982 is a total of = 18 pennies.
The mass of each penny for pre 1982 = 3.1
Therefore the total weight for penny for pre 1982
= 18×3.1
= 55.8
The sample of penny for post 1982 is a total of = 32 pennies.
The mass of each penny for post 1982 = 2.5
Therefore the total weight for penny for post 1982
=32×2.5
= 80
The weighted average
= 55.8+80/2
= 135.8/2
= 67.9g
learn more about mass here:
https://brainly.com/question/14156113
#SPJ1
Arrange the following order of increasing intermolecular forces: methane (ch4), propane (c3h8), butane (c4h10).
Answers
Order of increasing intermolecular forces will be CH4 < C3H8 < C4H10.
What is meant by intermolecular forces ?The electromagnetic forces of attraction or repulsion that operate between atoms and other types of nearby particles, such as atoms or ions, are examples of intermolecular forces (IMFs), also known as secondary forces. In comparison to intramolecular forces, which keep a molecule together, intermolecular forces are weak. For instance, the forces between adjacent molecules are substantially less than the covalent bond, which involves exchanging electron pairs between atoms.Both sets of forces are crucial components of the force fields that molecular mechanics frequently employs.Macroscopic observations that show the existence and operation of forces at the molecular level serve as the starting point for the study of intermolecular forces.Learn more about intermolecular forces refer to :
https://brainly.com/question/2193457
#SPJ4
what is the heat of reaction H2 + 1/3N2 2/3NH3
Answers
1/3N(g)+H(g)—>1/3NH3(g)
What term describes the lowest temperature to which air can be cooled at constant pressure before saturation occurs?
Answers
The minimum temperature that air must be chilled to in order to reach saturation at or below 0°C (32°F).
Is the temperature to which air must be cooled at constant pressure to reach saturation?The dew point is the temperature that must be reached while maintaining pressure for the air to become saturated, or for the relative humidity to reach 100%.
Simply said, the boiling point is also known as the saturation temperature. The term "saturation" refers to the temperature that a liquid must reach in order to boil and transition into the vapour phase based on its saturation pressure.
Intensity and chroma are other names for saturation. It speaks to the color's predominant hue. The "pure" hues are found at the outermost portion of the hue wheel. The hue we are using to describe the colour predominates less and less as you travel towards the centre of the wheel.
To learn more about saturation refer to:
https://brainly.com/question/601501
#SPJ4
How did Wegener work out what Pangea looked like?
Answers
Answer:
ok so basicalkly he lloked at how the subdiction zone formed and where previus conintal drift occured
Explanation:
2) a chemist combines 122.0 kg of ammonia with 211.4 kg ofcarbon dioxide, and obtains 185.1 kg of urea.a) determine the limiting reactant.b) determine the theoretical yield of urea. (answer: 215.3 kg)c) determine the percent yield for the reaction. (answer: 86.0%)d) how many kg of the excess reactant is left? (answer: 53.5 kg)
Answers
A. Ammonia is the limiting reactant.
B. Theoretical yield of urea is 215.3 kg.
C. Percent yield for the reaction is 86.0%
D. The mass of the excess carbon dioxide left is approximately 54.1 kg.
a) To identify the limiting reactant, we should compare the amount of products formed from each reactant. The chemical equation for the formation of urea \((NH_2CONH_2)\) by combining ammonia \((NH_3)\) and carbon dioxide \((CO_2)\) is as follows:
\(2 NH_3 + CO_2 - > NH_2CONH_2 + H_2O\)
The stoichiometry of the balanced equation indicates that the ratio of ammonia to urea is 2:1.
We can find the number of moles for each reactant using the following masses:
Moles of ammonia = 122.0 kg / 17.03 g/mol = 7.17 mol
Moles of carbon dioxide = 211.4 kg / 44.01 g/mol = 4.80 mol
It takes 14.34 moles of ammonia to react completely with the available carbon dioxide because the ratio of ammonia to urea is 2:1. But the amount of ammonia we have is less than we need - only 7.17 mol. As a result, ammonia is the limiting reactant.
b. Based on the limiting reactant, it is possible to calculate the theoretical yield of urea. We can use the moles of ammonia, which is the limiting reactant, to calculate the moles of urea:
Moles of urea = 7.17 mol / 2 = 3.58 mol
We can determine the theoretical yield of urea using the molar mass of urea (60.06 g/mol) as a starting point:
Theoretical yield of urea = 3.58 mol * 60.06 g/mol = 215.3 kg
C. The actual yield (185.1 kg) is calculated by dividing it by the theoretical yield (215.3 kg), then multiplying the result by 100%.
Percent yield = (185.1 kg / 215.3 kg) * 100% = 86.0%
D. We can calculate the amount of non-limiting reactant that has not reacted yet to determine the excess reactant. Since ammonia is the limiting reactant, we must determine how much excess carbon dioxide there is:
Moles of excess carbon dioxide = Moles of carbon dioxide initially - Moles of carbon dioxide used
= 4.80 mol - (7.17 mol / 2) = 1.23 mol
We can determine the mass of excess carbon dioxide using the molar mass of carbon dioxide (44.01 g/mol):
Excess carbon dioxide = 1.23 mol * 44.01 g/mol = 54.1 kg
Therefore, the mass of the excess carbon dioxide left is approximately 54.1 kg.
Learn more about Limiting reactant, here:
https://brainly.com/question/10090573
#SPJ4