What kind of reaction occurs when the overall entropy change is negative?
endothermic
exothermic
reversible
irreversible
Answers
The kind of reaction that occurs when the overall entropy change is negative is endothermic (option A).
What is an endothermic reaction?Endothermic reaction is a chemical reaction that absorbs heat energy from its surroundings.
In an endothermic reaction, the external entropy (entropy of the surroundings) decreases.
If a reaction is endothermic (H positive) and the entropy change (∆S) is negative (less disorder), the free energy change is always positive and the reaction is never spontaneous.
Learn more about endothermic reaction at: https://brainly.com/question/23184814
#SPJ1
Related Questions
A 1.00 L buffer solution is 0.250 M in HF and 0.250 M in LiF. Calculate the pH of the solution after the addition of 0.150 moles of solid LiOH. Assume no volume change upon the addition of base. The K a for HF is 3.5 × 10 -4.
3.46
4.24
3.63
2.85
4.06
Answers
The pH of the buffer solution after the addition of 0.150 moles of solid LiOH is 3.46, as calculated using the Henderson-Hasselbalch equation.
What is Buffer Solution?
A buffer solution is a type of solution that resists changes in pH when small amounts of acid or base are added to it. It is a solution made up of a weak acid and its conjugate base or a weak base and its conjugate acid, in approximately equal amounts.
Since 0.150 moles of LiOH are added to the buffer solution, they will react with an equal number of moles of HF, producing the same number of moles of LiF. Thus, the final concentrations of HF and LiF in the buffer will be:
[HF] = (0.250 mol/L x 1.00 L - 0.150 mol) / 1.00 L = 0.100 M
[LiF] = 0.250 mol/L + 0.150 mol / 1.00 L = 0.400 M
Next, we need to calculate the new pH of the buffer solution after the addition of LiOH. Since we have a weak acid-strong base buffer, we will need to use the Henderson-Hasselbalch equation:
\(pH = p_{Ka} + log([A-] / [HA])\)
where pKa is the dissociation constant of HF, [HA] is the concentration of the weak acid (HF), and [A-] is the concentration of the conjugate base (LiF).
The pKa of HF is given as 3.5 x\(10^{-4}\), so we can calculate the value of Ka:
\(Ka = 10^{-p_{Ka}} = 2.24 x 10^{-4}\)
Now we can substitute the values into the Henderson-Hasselbalch equation:
pH = 3.46 + log(0.400 / 0.100) = 3.46 + 0.602 = 4.06
Therefore, the pH of the buffer solution after the addition of LiOH is 3.46.
Learn more about Buffer Solution, visit;https://brainly.com/question/8676275
#SPJ4
How many significant figures does 5.750 have?
Answers
Answer:
4
Explanation:
Element X has two isotopes. The first isotope has a natural abundance of
70% and has a mass of 50 amu. The second isotope has a natural
abundance of 30% and has a mass of 54 amu. Is the average mass
going to be closer to 50 or 54 amu and why?
Answers
The average atomic mass is calculated on the basis of the average mass of all the isotopes present in the nature. Here the average mass is closer to 50 amu.
What is isotope?The isotopes are defined as the chemical elements which have the same atomic numbers but different mass numbers. The number of protons or electrons in them are same but the number of neutrons will be different.
₅₀X = 70% = 0.70
₅₄X = 30% = 0.30
Average atomic mass = (50 × 0.70) + (54 × 0.30) = 51.2
Thus the average atomic mass is closer to the first isotope of mass 50 amu. Because of the higher percent abundance of this isotope.
To know more about average atomic mass, visit;
https://brainly.com/question/14250653
#SPJ1
What is the number of moles of glucose (C₆H₁₂O₆) in 0.500 L of a 0.40 M solution?
Answers
Answer:
0.20 moles
Explanation:
In order to solve this problem it is necessary to keep in mind the definition of molarity:
Molarity = moles / litersIf we input the data given by the problem we're left with:
0.40 M = moles / 0.500 LMeaning that we can proceed to calculate the number of moles:
moles = 0.40 M * 0.500 Lmoles = 0.20 molesThe number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution is 0.2 moles.
Given the following data:
Molarity of solution = 0.40 MVolume of solution = 0.500 LTo determine the number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution:
Mathematically, the molarity of a solution is given by the formula:
\(Molarity = \frac{number\;of\;moles}{Volume \;in\;liters}\)
Making number of moles the subject of formula, we have:
\(Number\;of\;moles = Molarity \times Volume\)
Substituting the given parameters into the formula, we have;
\(Number\;of\;moles = 0.40 \times 0.500\)
Number of moles = 0.2 moles.
Read more: https://brainly.com/question/13750908
Compare the ir spectra for 9-anthraldehyde and that of your product. What evidence allows you to conclude that your product is trans-9-(2-Phenylethenyl)-anthracene? I don't have the spectrum for the 9-anthraldehyde, but here's the spectrum for my product.
Answers
The comparison of IR spectra between 9-anthraldehyde and the product, along with specific evidence, allows us to conclude that the product is trans-9-(2-Phenylethenyl)-anthracene.
What evidence supports the conclusion?To determine if the product is trans-9-(2-Phenylethenyl)-anthracene, a comparison of the IR spectra of 9-anthraldehyde and the product is necessary. While the provided spectrum is for the product, we can discuss the general evidence that would support the conclusion.
1. Absence of aldehyde peak: The IR spectrum of 9-anthraldehyde would show a strong absorption peak around 1700-1750 cm^-1 corresponding to the C=O stretching of the aldehyde group. In the product spectrum, the absence of this peak would indicate the absence of the aldehyde group.
2. Presence of alkene peaks: The product, trans-9-(2-Phenylethenyl)-anthracene, contains a double bond. The IR spectrum would exhibit characteristic peaks around 1600-1680 cm^-1 for the C=C stretching vibrations, indicating the presence of the alkene group.
3. Similarity in aromatic regions: Both 9-anthraldehyde and the product contain aromatic systems. Comparing the regions of the spectrum corresponding to aromatic C-H stretching vibrations (around 2900-3100 cm^-1) and aromatic C-C stretching vibrations (around 1400-1600 cm^-1) can reveal similarities in these regions, supporting the conclusion.
While the provided spectrum for the product is not available, comparing it to the expected IR features of trans-9-(2-Phenylethenyl)-anthracene would provide further confirmation.
Learn more about evidence
brainly.com/question/33111254
#SPJ11
A statement that explains, asserts, or predicts on the basis of statements (known as reasons) that are offered as evidence for it is called
Answers
A statement that explains, asserts, or predicts on the basis of statements (known as reasons) that are offered as evidence for it is called an argument.
An argument is a logical and structured presentation of a claim or assertion supported by reasons or evidence. It aims to persuade or convince others of the validity of the claim.
The main components of an argument include the claim itself, the reasons or evidence provided to support the claim, and the logical relationship between the claim and the reasons.
In an argument, the claim is the main statement being made, while the reasons serve as the supporting statements or evidence that back up the claim. The reasons provide logical connections and justification for accepting the claim as true or valid. By presenting coherent and persuasive reasoning, an argument seeks to establish the credibility and validity of the claim being made.
Learn more about claim here:
https://brainly.com/question/30296161
#SPJ4
How many moles of glucose are dissolved in 250 mL of a 2.67 M solution?

Answers
0.67 moles of glucose are dissolved in 250 mL of a 2.67 M solution.
Thus, The name "glucose" is derived from the Greek word for "sweet." Your body uses this particular sort of sugar, which it obtains from the food you consume, as fuel.
It is referred to as blood glucose or blood sugar when it passes through your bloodstream to reach your cells. The hormone insulin transports glucose from the blood into the cells for use as fuel and storage. Blood glucose levels are higher in diabetics than in healthy people.
Either their cells don't react to insulin as insulin should or they don't have enough insulin to get it through. Long-term high blood glucose levels can harm your kidneys, eyes, and other organs.
Thus, 0.67 moles of glucose are dissolved in 250 mL of a 2.67 M solution.
Learn more about Glucose, refer to the link:
https://brainly.com/question/13555266
#SPJ1
the party guy, mr. balloon, is making animal figures out of long thin balloons that he twists into different shapes. assume that the balloons are of uniform thickness, and that twisting them has a negligible effect on the volume of gas inside them. therefore, the length of a balloon is proportional to its volume. mr. balloon makes a doggie balloon out of a 2.0 meter balloon, and a butterfly out of a 1.5 meter balloon. the butterfly contains 3.5 moles of gas. how many moles are in the doggie balloon?
Answers
4.68 moles are their in 2m gas balloon.
As we know that,
1.5 m balloon contains 3.5 moles of gas
1 m balloon = \(\frac{3.5}{1.5} moles\)
1 m balloon = 2.34 moles
For 2 m balloon,
2 * 2.34 = 4.68 moles of gas are their.
A pure gas may consist of individual atoms (such as a noble gas like neon), elemental molecules made of a single type of atom (such as oxygen), or complex molecules made of several different types of atoms (e.g. carbon dioxide). There are many different pure gases in a gas mixture like air. The great separation of the individual gas particles is what sets gases apart from liquids and solids. A colorless gas becomes invisible to a human observer due to this gap.
To know more about gas click here:
https://brainly.com/question/18124975
#SPJ4
Which product is made using bacteria?
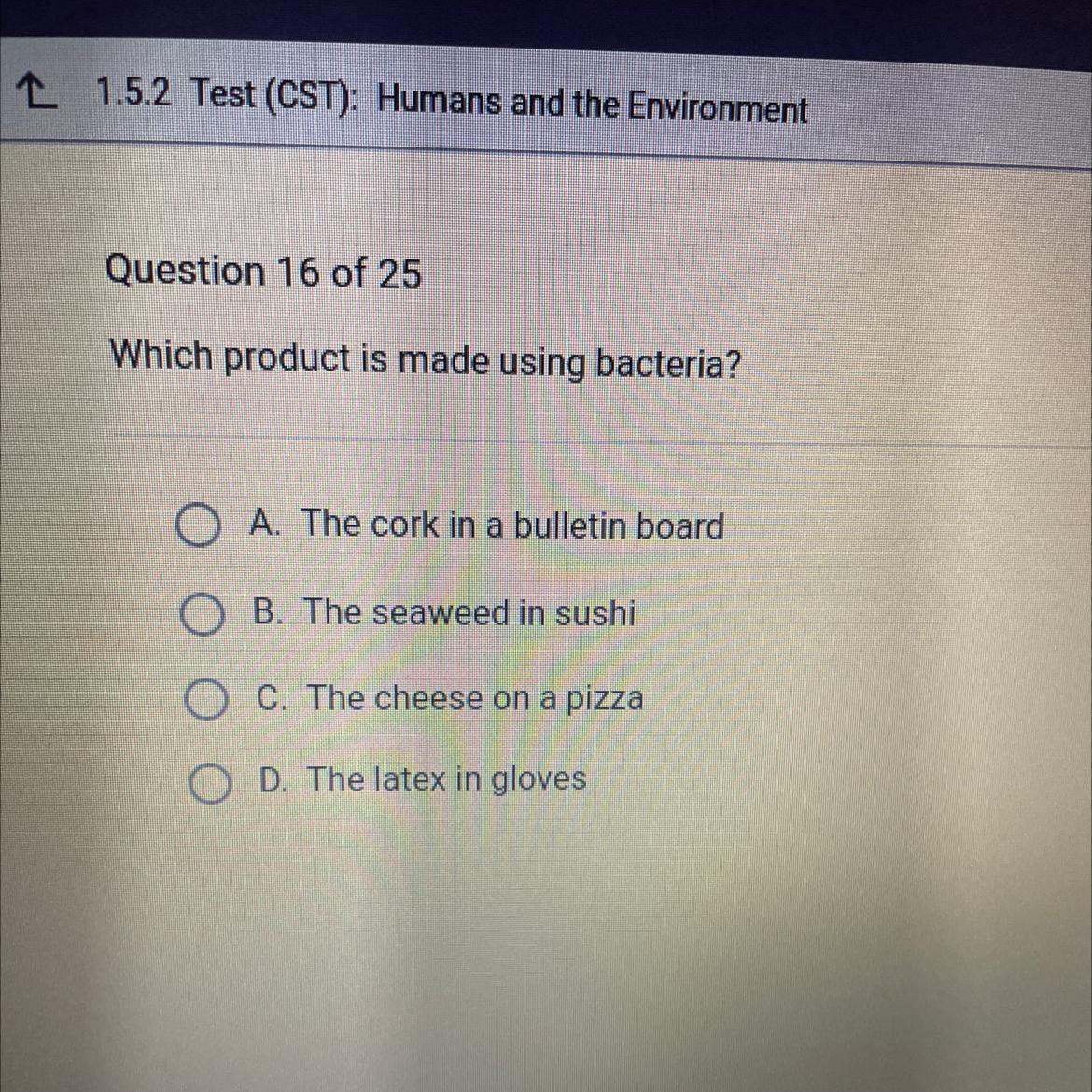
Answers
Answer:
C would be the answer
Explanation:
What is the molality of a solution prepared by dissolving 225 mg of glucose (C6H12O6) in 5.00 mL ofethanol (density = 0.789 g/mL)
Answers
= 0.316 mol/kg
Step-by-step explanation:
Molality is defined as the number of moles of solute per kilogram of solvent. We need to find the molality of the solution prepared by dissolving 225 mg of glucose in 5.00 mL of ethanol.
First, we need to convert the mass of glucose to moles:
Molar mass of glucose (C6H12O6) = 180.16 g/mol
Mass of glucose = 225 mg = 0.225 g
Number of moles of glucose = mass/molar mass = 0.225 g/180.16 g/mol = 0.00125 mol
Next, we need to calculate the mass of ethanol used in the solution:
Volume of ethanol = 5.00 mL
Density of ethanol = 0.789 g/mL
Mass of ethanol = volume x density = 5.00 mL x 0.789 g/mL = 3.945 g
Now we can calculate the molality of the solution:
Molality = moles of solute / mass of solvent in kg
Mass of solvent in kg = mass of ethanol / 1000 = 3.945 g / 1000 = 0.003945 kg
Molality = 0.00125 mol / 0.003945 kg = 0.316 mol/kg
Therefore, the molality of the solution prepared by dissolving 225 mg of glucose in 5.00 mL of ethanol is 0.316 mol/kg.
How many grams are in 4.2 X 10^2 moles of bromine?
Answers
Answer:
The answer is below:
Explanation:
3355.968
Calculate the maximum wavelength of light capable of dissociating the i–i bond in one molecule of iodine if the bond energy, or bond dissociation energy, is 153 kj/mol.
Answers
The maximum wavelength of light capable of dissosciating i-i bond in one molecule of iodine is 0.77m.
In dissociation, are bonds broken?The energy needed to break a bond and create two atomic or molecular fragments, each containing one of the original shared pair of electrons, is known as the bond dissociation energy.
What would be the maximum wavelength to dissosciate i-i bond?The energy required to break one i-i bond = bond energy per mol/ avagudro's number.
= 119.255x 10^3/ 6.023 x 10^23 J
Let the wavelength of the photon required to break one i-i bond be lambda .
Lambda = hc/E = 6.6x10^-34x3x10^8 x 6.023 x 10^23/ 153 x 10^3
=119.2554 x 10^-3/ 153 x10^3= 0.77 m
To know more about wavelength and dissosciating energy visit:
brainly.com/question/9350992
#SPJ4
What is a solution?
Answers
Answer
there are to answers one is an answer to a problem or a mixture of chemicals
Explanation:
Answer:
a liquid mixture in which the solute is uniformly distributed within the solvent.
Explanation:
Cooking an egg is one type of process, while the formation of snow is another. Which answer best explains which is exothermic and which is endothermic? Cooking an egg is endothermic because the egg gains heat from the surrounding Formation of snow is exothermic because water loses heat to the surrounding wh becomes snow. Cooking an egg is exothermic because the egg loses heat to the surroundings. Formation of snow is endothermic because water gains heat when it forms snow
Answers
Cooking an egg is endothermic because the egg gains heat from the surrounding. The formation of snow is exothermic because water loses heat to the surroundings when it becomes snow.
The endothermic process refers to the process in which heat is absorbed, while the exothermic process refers to the process in which heat is released.
The egg receives/absorbs heat from a heat source,(e.g. the stove), which raises the temperature of the egg and causes it to cook, making it an endothermic process.
On the other hand, heat is released from the water molecule when the water vapor present in the atmosphere condenses and freezes to form snow. Therefore, the formation of snow is exothermic.
Read more about endothermic and exothermic processes on:
https://brainly.com/question/30624362
Cooking an egg is endothermic because the egg gains heat from the surroundings, while the formation of snow is exothermic because water loses heat to the surroundings, resulting in the formation of snow.
In terms of energy transfer, an endothermic process absorbs heat from the surroundings, while an exothermic process releases heat to the surroundings. When cooking an egg, heat is applied to the egg through methods such as boiling, frying, or baking. The egg absorbs heat from the surrounding environment to cook and undergo chemical and physical changes. As a result, cooking an egg is an endothermic process because the egg gains heat from its surroundings.
On the other hand, the formation of snow occurs when water vapor in the atmosphere condenses into ice crystals. This process is exothermic because water vapor loses heat to the surroundings, causing the water molecules to slow down and form a solid structure. The release of heat during this phase transition results in the formation of snow. Therefore, the correct explanation is that cooking an egg is endothermic because the egg gains heat from the surroundings, while the formation of snow is exothermic because water loses heat to the surroundings, leading to the formation of snow.
Learn more about molecules here: https://brainly.com/question/14130817
#SPJ11
4. What trend in atomic radius occurs down a group on the periodic table?
rind on the periodic table?
Answers
Answer:Atomic radius gets bigger
Explanation:
Atomic radius bigger because not only do the atoms have more and more protons and neutrons, and thus more mass in general, there is also stronger shielding affect. Shielding affect is when electrons closer to the nucleus block the positive charge from reaching electrons farther from the nucleus, and thus those far electrons are not drawn towards the nucleus as strongly, and spread out more, increasing atomic radius.
pls help...
You are a forensic scientist working on a murder case. You have taken the blood sample from the crime scene and put it through Electrophoresis and Southern blotting. What step do you need to take next? A. Add ethanol B. Add restriction enzymes C. Add minisatellites D. Add radioactive probes
Answers
C. Add minisatellites
Explanation: hope this helps
Add minisatellites. Hence, option C is correct.
What is Electrophoresis?Electrophoresis is a laboratory technique used to separate DNA, RNA or protein molecules based on their size and electrical charge.
You are a forensic scientist working on a murder case. You have taken the blood sample from the crime scene and put it through Electrophoresis and Southern blotting. you need to add minisatellites.
Hence, option C is correct.
Learn more about Electrophoresis here:
https://brainly.com/question/13949940
#SPJ2
EXTRA POINTS.
WILL MARK BRAINIEST.
How many grams of AgCl are produced from 167 grams of AgNO3?
Answers
Answer:
1+2=5
Explanation:
1+2=5
put 20 ml of water into a 100 ml graduated cylinder. add 3g salt. cover with parafilm and mix until the salt dissolves. a. what is the final volume of the solution?
Answers
The final volume of the solution is 103 mL. This is because the salt will take up some volume when it dissolves, and the parafilm will add a small amount of additional volume.
Chemically speaking, when the salt dissolves in the water, a process known as dissolution occurs. This involves the breaking of the ionic bonds between the sodium and chloride ions of the salt, allowing them to move freely in the water.
The parafilm will also add a small amount of additional volume, as it is not completely watertight and will allow a small amount of water vapor to escape.
Learn more about dissolution:
https://brainly.com/question/16818744
#SPJ4
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure

Answers
The answer to your question is:
Psi = 14.7Atm = 1kPa = 101.3 What is Standard Atmospheric Pressure?The definition of the standard atmosphere's unit of pressure is 101325 Pa. It is occasionally used as a standard pressure or a reference pressure. It roughly corresponds to the average atmospheric pressure at sea level on Earth.
Psi: means "Pounds of force per Square Inch of area " an is a unit of pressure.
Atm: means atmosphere and is a unit of pressure defined as 101325 Pa.
kPa: is defined as the force of 1 Newton applied over one square meter.
( 101325 Pa = 101.3 kPa)
Then
Psi = 14.7Atm = 1kPa = 101.3Read more about atmospheric pressure here:
https://brainly.com/question/19587559
#SPJ1
where is the chemical energy that is produced in the light dependent reaction, used first in the plant?
Answers
The stroma is the place where light-dependent reactions in photosynthesis occur in plants.
Photosynthesis is said to be the method by which plants make their food by using the sun, air, and carbon dioxide.
The light-independent reaction in photosynthesis is referred to as the Calvin cycle and takes place in the stroma which is between the thylakoid membrane and the chloroplast membrane and does not require light, so it is called the light-independent reaction in photosynthesis. During this phase, energy from NADPH and ATP molecules is used to modulate carbohydrate molecules such as glucose from carbon dioxide.
Learn more about the light-independent reaction at https://brainly.com/question/26999184.
#SPJ4
When molybdate (MoO3) and Zinc (Zn) are heated together they react in this equation:
3Zn + 2MoO3 → Mo2O3 + ZnO
What is the mole ratio of Zn to Zno?
Answers
Answer: The mole ratio of Zn to ZnO is 3: 3.
Explanation:
According to law of conservation of mass, mass can be neither be created nor be destroyed. The mass on reactant side must be equal to the mass on product side. Thus the atoms of each element on both side of the reaction must be same.
The balanced chemical reaction is:
\(3Zn+2MoO_3\rightarrow Mo_2O_3+3ZnO\)
Here 3 moles of Zn combine with 2 moles of \(MoO_3\) to give 1 mole of \(Mo_2O_3\) and 3 moles of \(ZnO\). Thus the mole ratio of Zn to ZnO is 3: 3.
Which organelle makes useable energy for the cell? What type of fuel did your ship need in order to work?
Answers
Answer:
Mitochondria -uses glucose!!
Explanation:
__________ measures the brightness of a color and ranges from 0% (black) to 100% (white).
Hue
Saturation
Lightness
Darkness
Answers
Lightness measures the brightness of a color and ranges from 0% (black) to 100% (white).
The brightness of a color refers to the intensity of its lightness or darkness. It is determined by the amount of light reflected by the color. Brightness is often used interchangeably with value or tone, which refer to the lightness or darkness of a color.
In color theory, brightness is often represented on a scale from 0 to 100, with 0 being completely black and 100 being completely white. Colors with higher brightness values appear lighter and colors with lower brightness values appear darker.
Brightness can also be affected by the saturation of a color, which refers to the intensity of its hue or pure color. A highly saturated color appears more vibrant and intense, while a less saturated color appears more muted.
Visit here to learn more about color theory brainly.com/question/26331602
#SPJ11
The unit of radioactive exposure is given by a. grams b. rems c. ppm d. ppb '
Answers
The unit of radioactive exposure is given by (b) rems. The rem (Roentgen equivalent man) is a unit of measurement used to quantify the amount of radiation dose absorbed by living tissue. It takes into account the biological effects of different types of radiation on human health.
The unit of grams (a) is a measure of mass and is not specifically related to radiation exposure.
PPM (c) stands for parts per million, which is a unit used to express the concentration of a substance in a mixture. It does not directly measure radiation exposure.
Similarly, PPB (d) stands for parts per billion and is also a unit of concentration, not radiation exposure.
Therefore, the correct unit for measuring radioactive exposure is (b) rems.
To know more about radioactive exposure refer here :
https://brainly.com/question/31701460#
#SPJ11
Additional bands within the spectra correspond to LMCT bands. Explain LMCT in this particular system by providing details about the possible ligands that can contribute and which d orbitals are available for the interaction.
Answers
The d orbitals involved are the lower-energy, empty orbitals of the metal center (e.g., dxy, dxz, dyz), which can accept the electron density from the ligands. This interaction results in the observed LMCT bands in the spectra.
In this particular system, the additional bands within the spectra correspond to LMCT (ligand-to-metal charge transfer) bands. LMCT occurs when there is an electron transfer from a ligand to a metal ion.
The possible ligands that can contribute to the LMCT bands in this system are those that have lone pairs of electrons and are able to coordinate with the metal ion.
Examples of such ligands include water, ammonia, and halide ions. The d orbitals that are available for interaction in this system depend on the metal ion present.
Generally, transition metals have d orbitals available for interaction. These orbitals can be used to accept the electrons transferred from the ligand during the LMCT process, resulting in the formation of a coordination complex.
The specific d orbitals involved in the interaction will depend on the geometry of the complex and the identity of the ligands involved.
Overall, the LMCT process plays an important role in the formation and stability of coordination complexes in this system.
Visit here to learn more about Orbitals:
brainly.com/question/6202988
#SPJ11
Element whose atoms have completed their outer energy level are the ____
Answers
Answer:
Noble gases
Explanation:
nobles gases have their outer energy level complete
Which two subatomic particles have the same mass but do not have the same charge?
A. None of these
B. Neutrons and quarks
C. Electrons and neutrons
D. Protons and neutrons
Answers
Answer:
d
Explanation:
b/ce The strong force effects produce a mass that is essentially independent of the kinds of quarks involved, so this mass contribution will be the same for the proton and neutron.
PLEASE SOMEONE ACTUALLY HELP ME!!!!!!!!!!
The particles of a substance stay
close together but slide past one another
as they move. When thermal energy is added to the substance, the particles
start to move independently of one another. What change in state has
occurred?
A. Solid to gas
B. Liquid to solid
C. Solid to liquid
D. Liquid to gas
SUBMIT

Answers
What is the percentage of Aluminum in Aluminum Sulfate?
Answers
Answer:
342. 15 g/mol is the mass
Al Aluminium 26.98g 2 atoms per mole = 15.7716%
S Sulfur 32.06g 3 atoms per mole = 28.1151%
O Oxygen 15.99g 12 atoms per mole = 56.1133%
Explanation:
Hope this helps
Answer:
15,7%
Explanation:
What is the percentage of Aluminum in Aluminum Sulfate?
Aluminum Sulfate
is Al2 (SO4)3
its molar mass is
(2 X 27) + (3 X 96) = 54 + 288 = 344
THE % Al IS (54 /344) X 100 = 15,7%
How many moles of carbon dioxide are produced from the complete combustion of 5.42 moles of ethanol? 2C2H6O +7 O2 ®4 CO2 + 6H2O
Answers
Answer: 10.84 moles of \(CO_2\) will be produced from complete combustion of 5.42 moles of ethanol
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number \(6.023\times 10^{23}\) of particles.
\(2C_2H_6O+7O_2\rightarrow 4CO_2+6H_2O\)
According to stoichiometry :
2 moles of ethanol produce = 4 moles of \(CO_2\)
Thus 5.42 moles of ethanol will produce=\(\frac{4}{2}\times 5.42=10.84moles\) of \(CO_2\)
Thus 10.84 moles of \(CO_2\) will be produced from complete combustion of 5.42 moles of ethanol