what is the theoretical yield of water vapor in liters that can be produced when 34 g of oxygen gas reacts with 6 grams of hydrogen gas at stp
Answers
The theoretical yield of water vapor that can be produced is 47.6 liters.
We need to consider the balanced chemical equation for the reaction:
\(2H_2 + O_2 - > 2H_2O\)
From the balanced equation, we can see that 2 moles of \(H_2\) react with 1 mole of \(O_2\) to produce 2 moles of \(H_2O\).
First, we need to convert the given masses of the reactants into moles:
Moles of \(O_2\) = \(mass / molar\ mass = 34 g / 32 g/mol = 1.0625 mol\)
Moles of \(H_2\) = \(mass / molar\ mass = 6 g / 2 g/mol = 3 mol\)
Based on the stoichiometry, 1 mole of \(O_2\) produces 2 moles of \(H_2O\). Therefore, the theoretical yield of water vapor would be:
Theoretical yield of \(H_2O\) = 2 * Moles of \(O_2\) = 2 * 1.0625 mol = 2.125 mol
At STP, 1 mole of any ideal gas occupies 22.4 liters. Therefore, the theoretical yield of water vapor in liters would be:
Theoretical yield of \(H_2O\) =\(2.125 mol * 22.4 L/mol = 47.6 L\)
To know more about theoretical yield, here
brainly.com/question/14966377
#SPJ4
Related Questions
How many aluminum atoms are found in 8.57 moles of aluminum?
Answers
Answer:
1.02 10 to the 23 power(or 100,000,000,000,000,000,000,000)
atoms of AL
Explanation:
ive had this question before
What is the name of the element hawing the chemical symbol k?
Answers
The name of the element symbol K will be potassium.
A material is said to be an element if all of its atoms contain the same number of protons, or if all of its atoms have identical atomic numbers. Chemical processes cannot break down elements because they might be the simplest chemically.
The name of the element with symbol K is potassium. It has atomic number 19 and has electronic configuration 2,8,8,1. It has 1 valence electrons that's why it is kept in group 1 of the periodic table.
Therefore, the name of the element will be potassium.
To know more about element.
https://brainly.com/question/13025901
#SPJ1
Write 3 equations that is:
Metal + Acid (ex: dense H2SO4, HNO3) -> salt + H2O + (NO2/ NO/ SO2/...)
ex: Cu + 2H2SO4 (dense) -> CuSO4 + 2H2O + SO2
Answers
Here are three equations representing the chemical reaction between a metal and an acid:
Zinc + 2HCl → ZnCl2 + H2
Iron + 2HNO3 → Fe(NO3)2 + H2O + NO
Magnesium + 2H2SO4 → MgSO4 + 2H2O + SO2
The three equations representing the reaction :
Zinc + 2HCl → ZnCl2 + H2In this reaction, zinc (metal) reacts with hydrochloric acid to form zinc chloride and hydrogen gas.
Iron + 2HNO3 → Fe(NO3)2 + H2O + NOIn this reaction, iron (metal) reacts with nitric acid to form iron(II) nitrate, water, and nitric oxide.
Magnesium + 2H2SO4 → MgSO4 + 2H2O + SO2In this reaction, magnesium (metal) reacts with sulfuric acid to form magnesium sulfate, water, and sulfur dioxide.
In each of these equations, the metal reacts with the acid to produce a salt, water, and sometimes additional products such as hydrogen gas (H2), nitrogen dioxide (NO2), nitrogen monoxide (NO), or sulfur dioxide (SO2), depending on the specific acid and reaction conditions.
For more such questions on chemical reaction
https://brainly.com/question/14106530
#SPJ8
Which statement about the number of protons and neutrons in a nuclear change is correct?
Responses
The total charge before and after the change remains the same, but the total atomic mass is different.
The relative numbers of protons and neutrons are the same before and after the change.
The relative numbers of protons and neutrons can differ before and after the change, but the total number of those particles remains the same.
The total atomic mass before and after the change remains the same, but the total charge is different.
Answers
Answer:
The correct statement about the number of protons and neutrons in a nuclear change is: "The relative numbers of protons and neutrons can differ before and after the change, but the total number of those particles remains the same."
Explanation:
In a nuclear change, the total number of protons and neutrons (also known as nucleons) remains constant, but the specific arrangement of those nucleons can change. This can be achieved through processes such as nuclear decay, where an unstable nucleus emits particles and becomes more stable, or through nuclear reactions, where two or more nuclei collide and rearrange to form new nuclei. In either case, the total number of protons and neutrons is conserved, but the specific numbers of protons and neutrons in the resulting nuclei can be different from the original nuclei.
It's important to note that the total atomic mass (also known as the atomic weight) and total charge of a nucleus can change as a result of a nuclear change. The atomic mass is determined by the total number of protons and neutrons in a nucleus, so it will be different if the relative numbers of those particles change. The total charge of a nucleus is determined by the number of protons, so it will be different if the number of protons changes.
How to write H12O6 in lewis?
Answers
The molecule H₁₂O₆ does not exist in nature. The formula H₁₂O₆ suggests the presence of 12 hydrogen atoms and 6 oxygen atoms.
However, such a configuration is highly unstable and does not conform to the principles of chemical bonding and stability. In a typical molecule of water (H₂O), two hydrogen atoms are bonded to one oxygen atom. The Lewis structure for water would represent this bonding arrangement. Each hydrogen atom contributes one valence electron, and oxygen contributes six valence electrons.
The Lewis structure for water would show two lines (representing covalent bonds) connecting the oxygen atom to each hydrogen atom. Additionally, the oxygen atom would have two lone pairs of electrons (represented by dots) surrounding it. It is important to note that the Lewis structure represents the valence electron arrangement in a molecule and provides insight into its bonding and geometry.
However, the Lewis structure alone may not capture the full 3D shape and molecular properties. In the case of H₁₂O₆, the presence of 12 hydrogen atoms and 6 oxygen atoms is highly unlikely due to the unfavorable electron configuration and excessive charge repulsion.
know more about hydrogen atoms here:
https://brainly.com/question/28499820
#SPJ8
Which statement is part of the atomic theory of both Democritus and Dalton?
All matter is made of atoms.
Atoms of the same element are identical.
Atoms of different elements can combine in whole number ratios.
Chemical reactions occur when atoms are separated, joined, or rearranged.
Answers
A compound of molecules results from the combination of atoms from the same or distinct elements. Reason: According to the atomic theory of matter: In 1808, it is declared by the British chemist John Dalton.
What distinguishes Democritus from Dalton's atomic theory?Dalton's atomic theory is founded on evidence, but Democritus had no supporting data for his idea. This is the primary distinction between the two theories' atomic theories.
Which claim is included in Dalton's atomic hypothesis of matter's composition?All matter is made up of indivisible atoms, according to the first component of his theory. The second aspect of the theory states that the mass and properties of every atom in a particular element are the same.
To know more about atomic theory of Dalton visit :-
https://brainly.com/question/4121965
#SPJ1
what is the ph of a 1.66×10-2 m solution of the strong acid hbr? hbr(aq) h2o(ℓ) → br-(aq) h3o (aq) [hbr]0 = 1.66×10-2
Answers
The pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr is 1.78. The pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr can be determined using the equation pH = -log[\(H_3O^+\)].
Since HBr is a strong acid, it will completely dissociate in water to produce \(H_3O^+\) and Br- ions. Therefore, the concentration of \(H_3O^+\) ions will be equal to the initial concentration of HBr, or 1.66×\(10^{-2}\) M.
Using the equation pH = -log[\(H_3O^+\)], we can plug in the concentration of \(H_3O^+\) ions to find the pH:
pH = \(-log(1.66.10^{-2}) = 1.78\)
Therefore, the pH of a 1.66×\(10^{-2}\) M solution of the strong acid HBr is 1.78.
Here you can learn more about pH calculation https://brainly.com/question/30255888
#SPJ11
(ii) Describe briefly one chemical test to distinguish dilute
HCI from dilute HNO3
Answers
Answer:
By adding, a few drops of AgNO3
Dilute HCl reacts with silver nitrate to give a white precipitate of silver chloride.
On the other hand there is no reaction observed when dilute Nitric acid is treated with silver nitrate solution. That is obvious
Reactions :-
HCl(aq) + AgNO3(aq) >>> AgCl(s) + HNO3(aq)
HNO3(aq) + AgNO3(aq) >>> No reaction + No precipitate
1. Antarctica is a frozen land, so cold and icy that no trees can grow there. Yet scientists have discovered fossils(remains preserved in rock) of ancient trees in Antarctica
What do you think this means
Answers
Answer:
This means that Antarctica once had a warmer climate.
Explanation:
Trees usually grow in warm climates, and Antarctica has little to no plant life.
How many atoms are present in 0.056 moles of Copper (Cu)?
Answers
I need question 8 plzzzz

Answers
Answer:
3?
Explanation:
Answer:
B has 3 atoms
trust me have done problems like these
if anybody sees this could they help me out?
Listed in the Item Bank are some important labels for sections of the image below. Match each label to the corresponding area it identifies in the image.
Silicon, Arsenic and Antimony
Can exchange electrons with each other to form ionic compounds
May have no luster
Tin, Calcium and Potassium
Hydrogen, Oxygen and Chlorine
Can be electrical conductors
Comprised of atoms
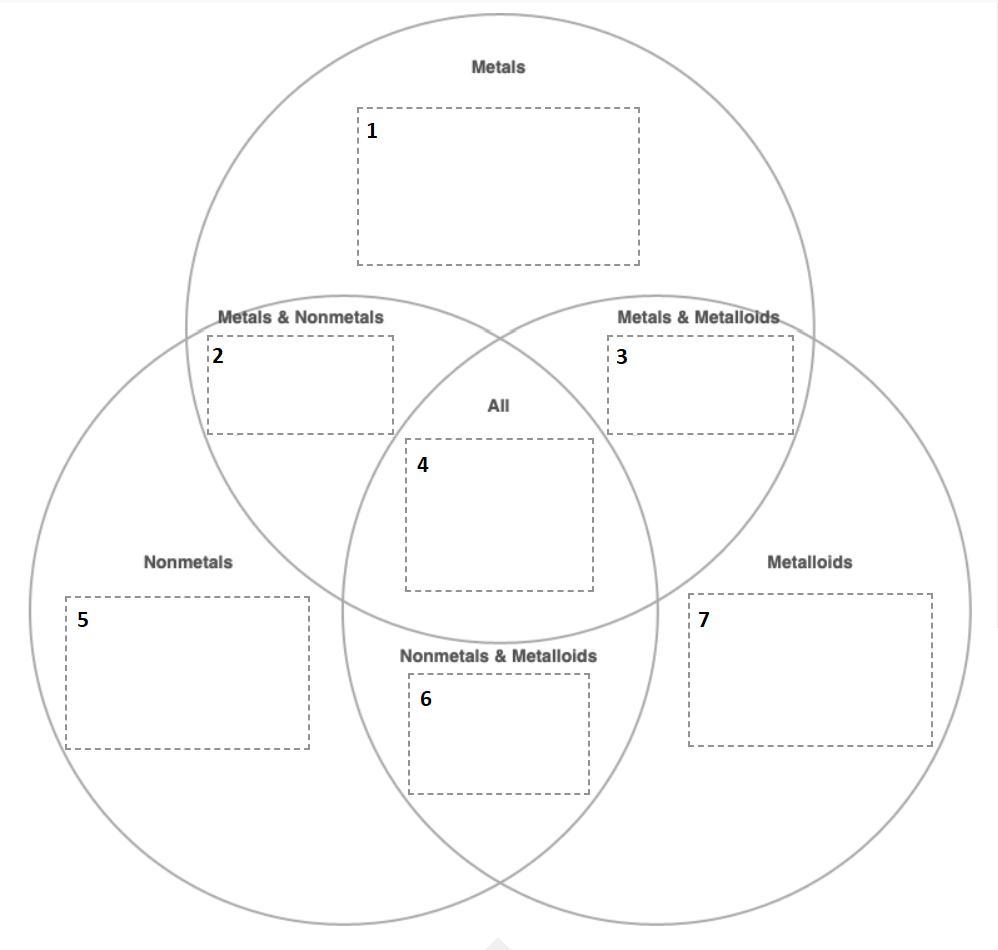
Answers
Based on the items listed in the Item bank, the corresponding area that identifies each item is given below:
Metalloids - Silicon, Arsenic, and Antimony
Metals and non-metals - Can exchange electrons with each other to form ionic compounds
Metalloids and Non-metals - May have no luster
Metals - Tin, Calcium, and Potassium
Non-metals - Hydrogen, Oxygen, and Chlorine
Metals and metalloids - Can be electrical conductors
All - Comprised of atoms
What are metals, metalloids, and non-metals?Metals are elements that form positive ions by the loss of electrons.
Metals are good electrical conductors and have a characteristic luster. Examples are Tin, Calcium, and Potassium.
Metalloids or semi-metals have intermediate properties of metals and non-metals. They conduct electricity but do not have any luster. Examples are Silicon, Arsenic, and Antimony.
Non-metals form negative ions by electron gain. They do not have a luster and are poor conductors of electricity.
Examples are Hydrogen, Oxygen, and Chlorine.
Learn more about metals, metalloids, and non-metals at: https://brainly.com/question/16757104
#SPJ1

Some bacteria live in the roots of plants like soybeans and peas.
Bacteria growing on plant roots.
What is the role of these bacteria in the nitrogen cycle?
to absorb nitrogen-containing compounds from the soil
to release free nitrogen into the atmosphere
to break down nitrogen-containing compounds in dead organisms
to convert free nitrogen into usable nitrogen
70 points!
Answers
Answer:
to release free nitrogen into the atmosphere. This is the answer.
Answer:
to convert free nitrogen into usable nitrogen
Explanation:
got it right on the test boi
Decomposition Reactions
1. sodium iodide →
2. magnesium nitride→
3. manganese (II) chloride >
4. potassium silicide>
Predict the reactants
Write out and solve the chemical equation
Answers
Answer:
NaI = Na + I2 (diatomic)
Mg3N2 = Mg + N2
MnCl2 = Mn + Cl2
K4Si = K + Si
Explanation:
First convert these written equations into chemical symbols.
Sodium iodide = NaI
Magnesium Nitride = Mg3N2
Manganese II Chloride = MnCl2
Potassium Silicide = K4Si
Then we predict the products by separating the elements.
NaI = Na + I2 (diatomic)
Mg3N2 = Mg + N2
MnCl2 = Mn + Cl2
K4Si = K + Si
However, notice that these are not balanced. If you want you can balance these.
what is the molecular geometry of the following and would you expect it to have a dipole moment? group of answer choices square planar, no octahedral, yes tetrahedral, yes octahedral, no square planar, yes
Answers
The molecular geometry of a given element is octahedral and it has no dipole moment. Therefore octahedral, No would be the correct answer.
The SF6 molecule has no dipole moment because each S−F bond dipole is balanced by one of equal magnitude pointing in the opposite direction of the other side of the molecule.
The three-dimensional configuration of the atoms that make up a molecule is known as molecular geometry. In addition to providing details about the molecule's overall shape, it also provides data on the bond lengths, bond angles, torsional angles, and any other geometrical factors that affect each atom's position.
Visit here to learn more about the molecule: https://brainly.com/question/1446104
#SPJ4

If I walked 45 minutes and burned 270 calories how many calories did. Burn in one minute please help
Answers
Answer:
6/cal per minute
Explanation:
To get the unit rate (in this case, calories per minute) just divide 270 by 45.
Which items can be classified as matter? Check all that apply.
Answers
Answer:
a globe
snow rain clouds
the instantaneous rate of a reaction can be read directly from the graph of molarity versus time at any point on the graph. the instantaneous rate of a reaction can be read directly from the graph of molarity versus time at any point on the graph. true false
Answers
The statement "the instantaneous rate of a reaction can be read directly from the graph of molarity versus time at any point on the graph." is False
Instantaneous rate of reaction is the rate at which a chemical reaction is occurring at a specific moment in time.
It is the slope of the tangent line at that particular point in time. The instantaneous rate of a reaction can be determined from a graph of concentration vs time.
We must draw a tangent line at the point in the graph that we are interested in, and the slope of that tangent line is the instantaneous rate of the reaction.
The rate can also be determined by finding the slope of a secant line over a very small time interval.
So, the given statement, “the instantaneous rate of a reaction can be read directly from the graph of molarity versus time at any point on the graph” is not true, and the correct answer is False.
To know more about molarity, refer here:
https://brainly.com/question/8732513#
#SPJ11
Why is an HCl molecule polar but a Cl2 molecule is nonpolar?
Answers
A polar substance is HCl. Due to its higher electronegative nature and unequal distribution of the bonding electrons with Hydrogen in the HCl molecule, Chlorine (Cl) atom (H). Yet, the molecules H2 and Cl2 are not polar since both atoms in the molecules have similar electronegativity.
As the bonds between the two atoms in the Cl2 Cl 2 molecule are identical, it is nonpolar. Hence, the electronegativity of the two atoms would not differ from one another. As a result of the nonpolar nature of the bond, the entire molecule is also nonpolar. The shared pair of electrons in a chlorine molecule (Cl2) is attracted to both chlorine atoms in an equal amount, creating a nonpolar connection.Chlorine is therefore a non-polar chemical. Yet, the bonding electrons in H-Cl are not distributed equally between the two atoms. As it has a linear symmetry and two chlorine atoms with equal electronegativity, Cl2 (chlorine) is nonpolar in nature. Because of the same charge distribution on both atoms and the molecule's zero dipole moment, the chlorine molecule is nonpolar.
Learn more about electronegativity here:
https://brainly.com/question/17762711
#SPJ4
The atomic models after dalton’s time included ideas about the atomic structure. which atomic model that shows the atomic structure is missing from this set? bohr’s model schrödinger’s model rutherford’s model thomson’s model
Answers
Which atomic model that shows the atomic structure is missing from this set?
Dalton’s model
In 1898, J. J. Thomson proposed the first of many atomic models to come as he made the proposition that the shape of an atom was like a sphere.
What is an Atomic Structure?This refers to the way and manner an atom is arranged and the things that are contained in it.
Hence, we can see that an atom contains:
ProtonsNeutronsNucleusTherefore, the atomic model that shows the atomic structure is missing from this set is Dalton's model.
Read more about atomic structures here:
https://brainly.com/question/13301755
#SPJ1
The complete question is:
The model of the atom has changed as scientists have gathered new evidence. Four models of the atom are shown below, but one important model is missing.
An image at left with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. An image at center left with a purple ball in the center surrounded by overlapping concentric black ovals, each with a small green ball on each of the 6 ovals. An image at center right with a large black cross in a purple circle with a black line around the purple, with 10 small green balls dispersed within the purple circle.
Which atomic model is missing from this set?
Bohr’s model
Dalton’s model
Rutherford’s model
Thomson’s model
Answer: B. schrodingers model
Explanation:
correct on edge
An understanding of periodic trends is impor-
tant because the trends
1. allow prediction of electron configurations
and bond orders.
2. allow compounds to be broken into their
elements.
3. allow confident analysis of the stock mar-
ket.
4. can be used to convert non-useful ele-
ments to useful ones.
5. relate to properties of elements and how
they may react.
Answers
An understanding of periodic trends is crucial as they relate to the properties of elements, their reactivity, and their behavior in chemical reactions. This knowledge aids in predicting electron configurations, determining bond orders, breaking compounds into elements, and utilizing elements effectively.
An understanding of periodic trends is important because the trends:
1. Allow prediction of electron configurations and bond orders: Periodic trends such as atomic size, ionization energy, and electron affinity provide information about the distribution and behavior of electrons in atoms. This knowledge helps in predicting electron configurations and determining bond orders in molecules.
2. Allow compounds to be broken into their elements: By understanding periodic trends, such as electronegativity and reactivity, we can predict how compounds can be broken down into their constituent elements through chemical reactions.
3. Allow confident analysis of the stock market: Periodic trends in the stock market are unrelated to the properties of elements and their reactivity. Therefore, periodic trends do not provide direct insights into stock market analysis.
4. Can be used to convert non-useful elements to useful ones: Periodic trends help in understanding the behavior of elements, which can be applied to develop processes for converting non-useful elements into useful ones through chemical reactions or refining techniques.
5. Relate to properties of elements and how they may react: Periodic trends provide information about various properties of elements such as atomic size, electronegativity, ionization energy, and reactivity. This knowledge helps in understanding the behavior of elements and predicting how they may react with other elements to form compounds.
To know more about the periodic trends refer here,
https://brainly.com/question/28729184#
#SPJ11
2014 Quantity Nuts 300 Meat 175 2015 Quantity Nuts 420 Meat 340 19 Using CPI what is the inflation rate from 2014 to 2015 if we assume 2014 is the base year? (enter your answer as a percentage and to 2 decimal places as needed) Price 9 19 Price 14
Answers
The inflation rate from 2014 to 2015, assuming 2014 as the base year, is 68.09%.
The inflation rate from 2014 to 2015, assuming that 2014 is the base year, can be determined using the Consumer Price Index (CPI). The CPI measures the cost of goods and services over time and is used to calculate inflation. The formula for calculating inflation rate using CPI is as follows: Inflation rate = ((CPI year 2 - CPI year 1) / CPI year 1) x 100To solve this problem, we first need to calculate the CPI for each year using the prices and quantities of nuts and meat.
Calculating CPI for 2014:CPI 2014 = (300 x $9) + (175 x $19) = $5,625 Calculating CPI for 2015:CPI 2015 = (420 x $9) + (340 x $14) = $9,460Using the CPI formula, we can calculate the inflation rate from 2014 to 2015:Inflation rate = ((CPI 2015 - CPI 2014) / CPI 2014) x 100= (($9,460 - $5,625) / $5,625) x 100= $3,835 / $5,625 x 100= 68.09% (to 2 decimal places) Therefore, the inflation rate from 2014 to 2015, assuming 2014 as the base year, is 68.09%.
To know more about rate visit:-
https://brainly.com/question/31892655
#SPJ11
Question 5
The energy produced by a generator can BEST be described as
A thermal
B chemical
C nuclear
D kinetic
E electrical
Answers
Answer:
E- Electrical
Explanation:
It converts mechanical energy to electrical energy
D kinetic
The generator in turn converts the kinetic energy of the rotor to electrical energy.
Hope it helped
What volume does 0.067 moles of He gas occupy at STP?
Answers
The volume of 0.067 moles of helium (He) gas at STP is equal to 1.5017 Liters.
Given the following data:
Number of moles = 0.067 molesScientific data:
Standard pressure = 1 atm.Standard temperature = 273 KIdeal gas constant, R = 0.0821 L⋅atm/mol⋅KTo determine the volume of 0.067 moles of helium (He) gas at STP, we would use the ideal gas equation:
\(V = n\frac{RT}{P}\)
Where;
P is the pressure.V is the volume.n is the number of moles of substance.R is the ideal gas constant.T is the temperature.Substituting the given parameters into the formula, we have;
\(V = \frac{0.067 \times 0.0821 \times 273}{1}\)
Volume, V = 1.5017 Liters
Read more on volume here: https://brainly.com/question/25290815
The hypothetical compound X has molar mass 84.91 g/mol and vapor pressure of 565 mmHg at 24°C. 50.0 g of coumpound X are introduced in a 15.0 L evacuated flask, sealed and left to rest until the liquid reaches equilibrium with its vapor phase. What will the mass of the liquid be once equilibrium is reached?
Answers
Answer:We can use the ideal gas law and the definition of vapor pressure to solve this problem.
First, we need to convert the vapor pressure from mmHg to atm:
565 mmHg = 0.743 atm
Next, we can use the ideal gas law to calculate the number of moles of gas in the flask:
PV = nRT
n = PV/RT
n = (0.743 atm) x (15.0 L) / [(0.08206 L·atm/mol·K) x (297 K)]
n = 0.436 mol
Since the molar mass of compound X is 84.91 g/mol, the mass of the gas in the flask is:
m = n x M
m = 0.436 mol x 84.91 g/mol
m = 37.0 g
Therefore, the mass of the liquid in the flask is:
50.0 g - 37.0 g = 13.0 g
So, the mass of the liquid once equilibrium is reached will be 13.0 g.
learn more about vapor pressure
https://brainly.com/question/29640321?referrer=searchResults
#SPJ11
A very large tank initially contains 100 L of pure water. Starting at time t=0 a solution with a salt concentration of 0.3 kg/L is added at a rate of 7 L/min. The solution is kept thoroughly mixed and is drained from the tank at a rate of 5 L/min. Answer the following questions. 1. Let y(t) be the amount of salt (in kilograms) in the tank after t minutes. What differential equation does y satisfy? Use the variable y for y(t). Answer (in kilograms per minute):
dt/dy = 2. How much salt is in the tank after 40 minutes? Answer (in kilograms):
Answers
1. The differential equation satisfied by y(t) is: dy/dt = 0.6 kg/min
The amount of salt in the tank after t minutes can be represented by the function y(t). We need to find the differential equation that y satisfies.
Initially, the tank contains 100 L of pure water, which means there is no salt in the tank. As time passes, a solution with a salt concentration of 0.3 kg/L is added at a rate of 7 L/min. The salt concentration in the tank will increase with the addition of this solution.
At the same time, the solution is drained from the tank at a rate of 5 L/min. This will result in a decrease in the salt concentration in the tank.
To find the differential equation satisfied by y(t), we need to consider the rate of change of salt in the tank.
Rate of change of salt in the tank = Rate of salt added - Rate of salt drained
The rate of salt added is given by the product of the concentration of the solution (0.3 kg/L) and the rate at which the solution is added (7 L/min). So, the rate of salt added = 0.3 kg/L * 7 L/min.
The rate of salt drained is given by the product of the concentration of the solution (0.3 kg/L) and the rate at which the solution is drained (5 L/min). So, the rate of salt drained = 0.3 kg/L * 5 L/min.
Therefore, the differential equation satisfied by y(t) is:
dy/dt = (0.3 kg/L * 7 L/min) - (0.3 kg/L * 5 L/min)
Simplifying the equation:
dy/dt = 2.1 kg/min - 1.5 kg/min
dy/dt = 0.6 kg/min
So, the differential equation satisfied by y(t) is:
dy/dt = 0.6 kg/min
2. The amount of salt in the tank after 40 minutes is 24 kilograms.
To find the amount of salt in the tank after 40 minutes, we can solve the differential equation.
dy/dt = 0.6 kg/min
Integrating both sides with respect to t:
∫dy = ∫0.6 dt
Integrating, we get:
y = 0.6t + C
To find the value of C, we can use the initial condition that the tank initially contains 100 L of pure water, which means there is no salt. So, at t = 0, y = 0.
Substituting these values into the equation:
0 = 0.6(0) + C
C = 0
Therefore, the equation becomes:
y = 0.6t
Now, we can find the amount of salt in the tank after 40 minutes by substituting t = 40 into the equation:
y = 0.6(40)
y = 24 kg
Learn more about differential equations from the given link:
https://brainly.com/question/1164377
#SPJ11
Products when starting alkyl halide is chiral and why?
Answers
When starting with a chiral alkyl halide, the products of a substitution reaction can be either achiral or chiral, depending on the reaction mechanism and the stereochemistry of the nucleophile.
In an SN1 reaction, the nucleophile attacks the carbocation intermediate from either side with equal probability, resulting in a racemic mixture of both R and S enantiomers.
In contrast, in an SN2 reaction, the nucleophile approaches the alkyl halide from the opposite side of the leaving group, resulting in an inversion of stereochemistry at the chiral center. This means that the product will be the opposite enantiomer of the starting material, resulting in a chiral product.
However, if the nucleophile used in the SN2 reaction is achiral, such as a hydroxide ion, the product will be racemic, as the reaction can occur with equal probability on both sides of the molecule.
In summary, the products of a substitution reaction starting with a chiral alkyl halide can be either achiral or chiral, depending on the reaction mechanism and the stereochemistry of the nucleophile.
To know more about nucleophile
brainly.com/question/28325919
#SPJ11
What is the coefficient of Fe3+ when the following equation is balanced in basic solution?
CN- + Fe3+ ? CNO- + Fe2+
A. 1
B. 2
C. 3
D. 4
E. 5
Answers
The coefficient of Fe3+ when the following equation is balanced in basic solution is B. 2.
How to balance the following equation?
CN- + Fe3+ ? CNO- + Fe2+
In basic medium, add one OH- ion to both sides for balancing the H+ ions. And the reaction becomes,CN- + Fe3+ + OH- → CNO- + Fe2+ + H2OUse the inspection method to balance the reaction.CN- + Fe3+ + OH- → CNO- + Fe2+ + H2O1 1 1 1 1 1+1 1 2 1The coefficients of Fe3+ and Fe2+ are 1 and 1, respectively, which implies that the transfer of one electron takes place from Fe3+ to CN-.
Therefore, the coefficient of Fe3+ is 1 when the given equation is balanced in the basic medium. However, we can't consider the coefficient of Fe3+ as 1 because this equation cannot be balanced without changing the coefficients of Fe3+ and Fe2+.Thus, we modify the equation by making the coefficients of Fe3+ and Fe2+ 2 and 3, respectively.CN- + 2Fe3+ + 3OH- → CNO- + 2Fe2+ + H2O
Therefore, the correct answer is B. 2.
To know more about coefficient click on below link :
https://brainly.com/question/6668768#
#SPJ11
plum-pudding model other name
Answers
Answer:
Thomson model of the atom
Explanation:
"plum pudding" from
raisins used to be called plums
distribution of electrons reminded many scientists of raisins
within Thomson's model's positively charged region of space
wikipedia
nuclearpowercom
What is the steric number of H2O2?
Answers
Answer: 1(2)+6(2)=14
Explanation:
Not sure, but maybe.