What is the relationship between the tendency of a main-group element to form a monatomic ion and its position in the periodic table? In what part of the table are the main-group elements that typically form cations? Anions?
Answers
Elements that tend to form monoatomic ions are either on the most right or the most left side of periodic table.
What is monoatomic ions ?Monatomic, which is a combination of the terms "mono" and "atomic" and refers to a single atom, is a term used in physics and chemistry. A monatomic gas is a gas in which atoms are not bonded to one another. This term is typically used to describe gases. All of the noble gases (helium, neon, argon, krypton, xenon, and radon) are examples at conventional temperatures and pressures, yet at sufficiently high temperatures, all chemical elements become monatomic in the gas phase (or very low pressure). Because a monatomic gas has no rotational or vibrational energy, its thermodynamic behavior is significantly simpler than that of polyatomic gases.
To learn more about monoatomic ions from the given link:
brainly.com/question/3842005
#SPJ4
Related Questions
How would you finish the last sentence? What would you say to Sherman so he understands that velocity and force are different?
Answers
Answer:
Because force is what causes things to move or change while volactiy is when you add more energy and the things volactiy will change.
Explanation:
how many modern periodic table contains
Answers
Answer:
118 elements in the periotic table
Explanation:
:)
When a solution becomes more acidic, does its pH increase or decrease? Explain up to 2 to 4 sentences.
Answers
Answer:
it decrease when a solution becomes more acidic which means that decrease in pH means increase..
Answer:
it's ph decreases.. meaning those with a small ph value are more acidic.forinstance one with a ph of 1 is more acidic than the one with a ph of 3.
I hope this helps
Water (H2O) is a liquid at room temperature (22 °C) while methane (CH4) is a gas. Which BEST explains the difference in their phases of matter at room temperature?
Answers
Answer:
Explanation:
Methanes boiling point would be higher than water's
Water is a liquid because particles are held close to one another by hydrogen bonds, whereas methane is a gas because the weak IMF lets particles to spread out.
What is methane ?Hydrocarbon methane ( CH4 ) is a main constituent of natural gas. Because methane is a greenhouse gas (GHG), its presence in the atmosphere has an impact on the planet's climate and temperature.
A range of anthropogenic (influenced by humans) and natural sources release methane into the atmosphere.
The significant difference in polarity between oxygen and hydrogen causes the water molecules to be dipolar. As a result, a strong hydrogen bond forms between the H and O of nearby water molecules, packing the water molecules so closely together that water is a liquid at ambient temperature.
Thus, water is a liquid because particles are held close to one another by hydrogen bonds, whereas methane is a gas because the weak IMF lets particles to spread out.
To learn more about methane, follow the link;
https://brainly.com/question/2127750
#SPJ2
Give an example of how knowledge of physical properties of matter can be used in everyday life
Answers
Understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
Knowledge of physical properties of matter is extremely important in everyday life as it helps us understand the nature of substances we come into contact with. One example is the use of boiling points in cooking. Different substances have different boiling points which determine the temperature at which they boil. This information is crucial in determining cooking times and ensuring that food is cooked properly.
For instance, water boils at 100 degrees Celsius, while sugar syrup boils at a much higher temperature. If the wrong temperature is used, food may be undercooked or overcooked, leading to undesired outcomes. Knowledge of physical properties also helps in choosing the right materials for different purposes, such as choosing heat-resistant materials for cooking.
In conclusion, understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
To know more about matter visit:
brainly.com/question/28487167
#SPJ11
For which salt in each of the following groups will the solubility depend on pH?
a.AgF,AgCl,AgBr b.Pb(OH)2,PbCl2b c.Sr(NO3)2,Sr(NO2)2 d.Ni(NO3)2,Ni(CN)2
Answers
Salt in each of the following groups will the solubility depend on pH is AgF, AgCl, and AgBr
Solubility depends on the pH of salts in the following groups: Group 1: The solubility of carbonates, hydroxides, and sulfates is a function of pH. Group 2: The solubility of sulfides, hydroxides, and sulfites is a function of pH.Group 3: The solubility of sulfides, carbonates, and hydroxides is a function of pH.Group 4: The solubility of sulfides, hydroxides, and sulfites is a function of pH.Group 5: The solubility of sulfides, hydroxides, and sulfites is a function of pH.Group 6: The solubility of sulfides, carbonates, and hydroxides is a function of pH.
A salt is formed from the reaction of an acid and a base. Salts can be made up of a variety of ions, which can affect their solubility in water. Salts containing anions that can act as weak bases are usually more soluble in acidic solutions than in basic solutions. Salts with anions that can act as strong bases are usually more soluble in basic solutions than in acidic solutions. AgF, AgCl, and AgBr are the salts in each of the groups mentioned above that will have solubility dependent on pH. They contain halide ions, which can act as weak bases. The solubility of these salts in water is thus determined by the pH of the solution.
Learn more about Solubility at brainly.com/question/29661360
#SPJ11
what function does sodium hydroxide serve in the aldol reaction? none of the answers shown are correct. sodium hydroxide acts as an enone in this aldol reaction. sodium hydroxide donates a hydroxyl group in the formation of the alcohol. the sodium hydroxide solution serves as the solvent for the reaction.
Answers
Sodium hydroxide plays a crucial role in facilitating the aldol reaction and promoting the formation of new carbon-carbon bonds.
Sodium hydroxide is a key component in the aldol reaction. One of its main functions is to serve as a strong base, which helps to deprotonate the alpha carbon of the carbonyl compound (such as an aldehyde or ketone) involved in the reaction. This deprotonation leads to the formation of an enolate intermediate. Sodium hydroxide also acts as a source of hydroxide ions, which can attack the carbonyl carbon of a second carbonyl compound, resulting in the formation of an aldol product. In addition to its role as a base and nucleophile, sodium hydroxide can also function as a solvent for the reaction. Overall, sodium hydroxide plays a crucial role in facilitating the aldol reaction and promoting the formation of new carbon-carbon bonds.
To know more about sodium hydroxide visit :
https://brainly.com/question/10073865
#SPJ11
Where does the second stage of cellular respiration
occur?
W
Х
Y
Z
Please hurry this is a test

Answers
Answer:
X is your answer Have a good day
Explanation:
The pyruvate molecules from glycolysis next enter the matrix of a mitochondrion. That's where the second stage of cellular respiration takes place. This stage is called the Krebs cycle. During this stage, two more molecules of ATP are produced
Answer:
z
Explanation:
How does an electric generator work?
Answers
Answer:
A conductor coil (a copper coil tightly wound onto a metal core) is rotated rapidly between the poles of a horseshoe type magnet. ... The magnetic field will interfere with the electrons in the conductor to induce a flow of electric current inside it.
Which is the correctly balanced equation? *
A Ca + HCl → CaCl2 + H2
B Ca + HCl → CaCl2 + H2
cCa + H2Cl2 → CaCl2 + H2
D Ca + 2HCl → CaCl2 + H2
PLEASE ANSWER I WILL GIVE BRAINLIEST
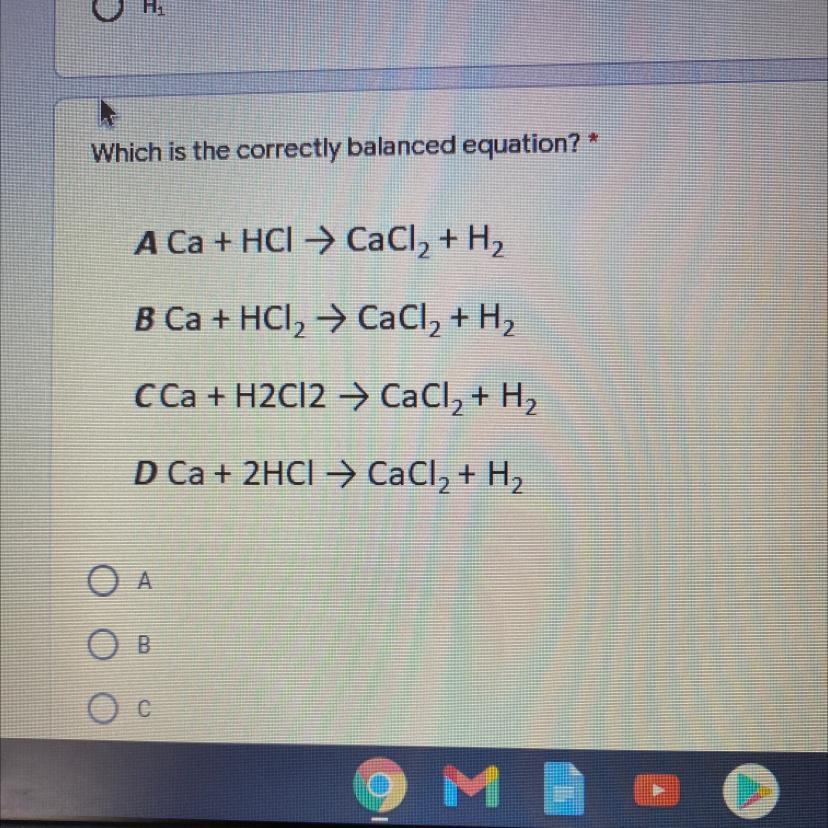
Answers
Answer:
Ca + 2HCl → CaCl2 + H2
Explanation:
This ia balanced equation on both side 1 Ca and 2 H and 2 Cl are present
Rank the following species in order of decreasing boiling point (highest to lowest): O3, N2, H2,CO2, O2
Answers
The following species can be listed in order of decreasing boiling point:
CO₂>O₃>N₂>O₂>H₂
The boiling point of gases depends upon the strength of the intermolecular forces of attraction acting between them and the molecular weight of the gaseous species.
CO₂ has polar bonds and also exhibits dipole - dipole interactions.
O₃ also has polar covalent bonds
O₂, N₂ and H₂ are non polar but have london dispersion forces as weak intermolecular forces.
Thus, the order of decreasing boiling point will be -
CO₂>O₃>N₂>O₂>H₂
Learn more about Boiling point, here:
https://brainly.com/question/2153588
#SPJ1
Which of the following statements about buffers is false? a) a buffer resists changes in pH upon addition of small amounts of strong acids and bases b) a buffer solution can react with either HjOor OH ions c) a buffer solution resists change in pH upon small dilutions d) a buffer always consists of a weak acid and a soluble ionic salt of the weak acid e) most body fluids contain natural buffer systems
Answers
The false statement about buffers is: d) a buffer always consists of a weak acid and a soluble ionic salt of the weak acid.
Buffers are solutions that can resist changes in pH when small amounts of acids or bases are added. They typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The weak acid and its conjugate base are present in equilibrium, allowing the buffer to accept or donate protons to maintain a relatively constant pH. Option d is false because a buffer can consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. It is not necessary for a buffer to always include a soluble ionic salt.
To learn more about buffers click here: brainly.com/question/31847096 #SPJ11
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Perform the following operationand express the answer inscientific notation.7.06x105 = 5.3x10-2][? ]x10!?)Coefficient (green)Exponent (yellow)Enter
![Perform the following operationand express the answer inscientific notation.7.06x105 = 5.3x10-2][? ]x10!?)Coefficient](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/JjIs2nMjTXVry0jMttVaLOs3WQJ7cjJr.jpeg)
Answers
To do this division, we can divide the power of ten and the leadin numbers separately:
\(7.06\times10^5\div5.3\times10^{-2}=\frac{7.06\times10^5}{5.3\times10^{-2}}=\frac{7.06}{5.3}\times10^{5-(-2)}=1.3320\ldots\times10^7\approx1.3\times10^7\)Give the equation with the elements in MgSO4 in their standard state as the reactants and MgSO4 as the product.
Answers
The balanced chemical equation for this reaction is:
2 Mg (s) + O₂ (g) → 2 MgO (s)
MgO (s) + SO₃ (g) → MgSO₄ (s)
The overall balanced chemical equation for the reaction is:
2 Mg (s) + O₂ (g) + SO₃ (g) → 2 MgSO₄ (s)
In this equation, the reactants are magnesium (Mg) and sulfur trioxide (SO₃) in their standard states, and the product is magnesium sulfate (MgSO₄) in its standard state.
However, burning magnesium in the air to create magnesium oxide and then reacting the oxide with sulfur trioxide gas to create magnesium sulfate are two potential ways to create MgSO4 from its component elements in their normal condition.
With an arrow separating the two sides of the equation, the reactants are written on the left and the products are written on the right. The coefficients in front of each species show how much of each material reacts or is created in relation to the other.
According to the rule of conservation of mass, a balanced chemical equation contains the same amount of atoms of each element on both sides of the equation.
learn more about MgSO₄ here
https://brainly.com/question/8691104
#SPJ1
the SI unit for forces is it a newton, force, unbalanced forces, balanced forces, net forces pick on I need you help
Answers
Answer: The SI unit for force is Newtons (N).
What happens when a wave hits an object?
the wave is reflected
the wave continues on
the wave is interfered with
the wave stops
Answers
explaination: in which, reflected stately happens when a wave strikes an object
EXERCISE 3: WHAT DOES pCO2 CHANGE? - When pCO
2
increases, the concentration of total CO
2
dissolved in water - When pCO
2
increases, the concentration of only CO
2
dissolved in water - When pCO
2
increases, the pH - Which form of dissolved CO
2
is most common in water? Ocean acidification is the decrease in pH due to increasing atmospheric CO
2
concentration.
2
. Choose the correct word option in the statements below: - An organism that needs CO
2
is likely to fare better / worse under ocean acidification. - An organism that needs HCO
3
- is likely to fare better/worse under ocean acidification. - An organism that needs CO
3
2−
is likely to fare better/worse under ocean acidification.
Answers
pCO2 is an important factor that affects various aspects of water chemistry and the impacts of ocean acidification. When pCO2 increases, the concentration of total CO2 dissolved in water also increases. This leads to changes in pH, which decreases due to increasing atmospheric CO2 concentration.
When pCO2 rises, the concentration of only CO2 dissolved in water increases. The dissolved CO2 forms carbonic acid, which contributes to the acidification of the ocean. This increase in CO2 affects the equilibrium between CO2, HCO3-, and CO3^2-, shifting it towards higher levels of dissolved CO2 and H+ ions, resulting in a lower pH.
In terms of the impacts of ocean acidification on different organisms, the effects can vary depending on their specific needs. An organism that requires CO2 is likely to fare better under ocean acidification since the increase in dissolved CO2 can provide them with a favorable environment. However, organisms that rely on HCO3- or CO3^2- may fare worse under ocean acidification, as the lower pH interferes with the availability of these carbonate ions, which are essential for shell formation and calcification in some marine organisms.
To know more about pCO2, click here, https://brainly.com/question/33500517
#SPJ11
Part 1: Other than distance from the sun, which factor affects the temperature of a planet?
Part 2: Compare the temperatures of two planets which are most likely affected by this factor. Use complete sentences for
your answer.
Answers
Answer:
part 1. minerals
part2. mars and earth the temperature is different by 0.5° Celsius
Explanation:
because the evaluation and factors of minerals are extremely affectively that the climate is likely to be affected
URGENTTTT
if S contributes a charge of -4, then what is the charge of the gold in AuS2?
Answers
The charge of the gold ion in AuS₂ is +4.
How to find a charge on a compound?The charge of the gold in AuS₂ can be determined by balancing the charges of the individual components. In this case, the sulfide (S) ion has a charge of -2, and there are two sulfide ions in AuS₂, so the total charge from the sulfide ions is -4. To balance this charge, the gold ion must have a charge of +4.
Therefore, the charge of the gold ion in AuS₂ is +4. This information can be used to write the balanced chemical equation for the formation of AuS₂, which would be represented as:
Au⁺⁴ + 2S⁻² → AuS₂
This equation shows that two sulfide ions with a charge of -2 are combining with a single gold ion with a charge of +4 to form the compound AuS₂.
Learn more on ionic charge here: https://brainly.com/question/22530066
#SPJ1
a five part per million standard has a percent transmission on a spectrometer of 34.780. what is the absorbance?
Answers
The absorbance of the sample with 34.780 percent transmittance is 3.54
What is transmittance?
Transmittance is the fraction of the incident light which passes through the sample and comes out successfully. In spectrometer, the detector detects the amount of light coming out of the sample and converts the reading into digital display. Absorbance is the fraction of the light which is being absorbed by the given sample.
The absorbance of the sample is,
A = 2 - log (-% T)
where, A = absorbance, %T = transmittance
A = 2 - log (-34.780) = 2 - (-1.54) = 3.54
Therefore, the absorbance of the sample having 34.780 percentage transmittance is 3.54.
To learn more about transmittance click on the link https://brainly.com/question/14919298
#SPJ4
Which statement is true about oxygen-17 and oxygen-18? A Their atoms have identical masses B They do not have the same number of protons C They have different numbers of electrons D They are isotopes of oxygen
Answers
Answer:
option d
Explanation:
isotope means atoms with different mass no. 17 and 18 written on the left represent mass number
this is because atoms can differ in mass no.
option a is incorrect as they have different mass no,hence,different massoption b is incorrect as atoms-oxygen-17 and oxygen 18-of same element oxygen cannot differ in no of protonsif they had different no of electons they would have a charge like -1,-2,etcoption d is correct,because atoms can differ in mass no. isotope means atoms with different mass no. 17 and 18 written on the left represent mass numberWhat is carbonization?
Answers
Answer:Carbonization is the conversion of organic matters like plants and dead animal remains into carbon through destructive distillation.
HOPE IT HELPS
Balance these equations
•Zn+HCl >> ZnCl2 + H2
•S8 + F2 >> SF8
Answers
Explanation:
Zn+2HCl>>>ZnCl2+H2S8+8F2>>>8SF8hope this helps you.
fungi have eukaryotic cells (large cells with a nucleus), like animals and plants
True or false?
Answers
Suppose a deep sea diver dives from the surface to 81 feet below the surface. He then dives down 13 more feet.
Find and interpret the sum to describe the diver's present depth.
Answers
Answer:
-94 feet.
Explanation:
A certain atom has a mass of 16 and an atomic number of 7. What element is this ?
Answers
Answer:
Nitrogen
Explanation:
According to the Periodic Table, the atom with a mass is 16 but the Atomic number is 7 for Nitrogen. That means that Nitrogen is an isotope.
g what is the overall balanced reaction when an aqueous solution of srcl2 reacts with an aqueous solution of na3po4?
Answers
Balanced reaction for an aqueous solution of SrCl₂ reacting with an aqueous solution of Na₃PO₄ is:
3SrCl₂ + 2Na₃PO₄ = Sr₃(PO₄)₂ + 6NaCl
What is a balanced chemical equation?A balanced chemical equation is where the number and charge of atoms of each type in the reaction is the same for both reactants and product sides. the mass and the charge should be same on both sides for a balanced reaction.
For the given equation:
SrCl₂ + Na₃PO₄ = Sr₃(PO₄)₂ + NaCl
Sr on both sides will be 3
Cl on both sides will be 6
Na on both sides will be 6
P on both sides will be 2
O on both sides will be 8
the balanced equation is, 3SrCl₂ + 2Na₃PO₄ = Sr₃(PO₄)₂ + 6NaCl
To know more about balanced chemical equation visit:
https://brainly.com/question/8062886
#SPJ4
15 pts! Explain how the absorption of energy (heating) affects the speed of the particles in a substance.
Answers
Answer:
See explanation
Explanation:
Temperature has been severally defined as the measure of the average kinetic energy of the molecules of a body. Heat is a form of energy that exists due to temperature difference.
Given a body whose molecules has a speed = (3RT/M)^1/2; this implies that for a given mass of the substance, the velocity of its particles depends on the temperature of the substance.
However, absorption of heat increases the temperature of the body. Therefore, when heat is absorbed, the temperature increases and the speed of the particles of a substance increases according to the relation above.
What do acids do in solution?

Answers
The answer should be B.....