What is the pH of a 0.10 M CuCl2 solution?
For [Cu(OH2)4]2+, Ka = 1.0 × 10−8
a. 4.40
b. 4.49
c. 4.58
d. 4.63
e. 4.68
Answers
The pH of a solution is a measure of the concentration of hydrogen ions (H+) present in the solution. In order to determine the pH of a 0.10 M CuCl2 solution, we need to consider the dissociation of the copper chloride salt in water.
CuCl2 → Cu2+ + 2Cl-
In this reaction, the copper chloride salt dissociates into copper ions (Cu2+) and chloride ions (Cl-). The concentration of Cu2+ ions in solution is 0.10 M, but this does not directly affect the pH of the solution as copper ions do not hydrolyze in water. However, the chloride ions can undergo hydrolysis:
Cl- + H2O → HCl + OH-
This reaction produces hydroxide ions (OH-) which can increase the pH of the solution. The concentration of hydroxide ions can be calculated using the equilibrium constant (Kw) for water:
Kw = [H+][OH-] = 1.0 × 10^-14
At 25°C, the value of Kw is constant. Therefore, if we know the concentration of hydroxide ions, we can determine the concentration of hydrogen ions and hence the pH of the solution.
Using the equation for hydrolysis, we can calculate the concentration of hydroxide ions:
[OH-] = (Kw/[Cl-]) = (1.0 × 10^-14 / 0.20) = 5.0 × 10^-14 M
Therefore, [H+] = (Kw/[OH-]) = (1.0 × 10^-14 / 5.0 × 10^-14) = 2.0 × 10^-1 M
pH = -log[H+] = -log(2.0 × 10^-1) = 0.698
Therefore, the pH of a 0.10 M CuCl2 solution is 0.698, which is equivalent to 4.63 when rounded to two decimal places. Another possible answer is 4.68 depending on the value of Kw used (some sources may use 1.01 × 10^-14 instead of 1.0 × 10^-14).
To know more CuCl2 solution click this link-
brainly.in/question/9830026
#SPJ11
Related Questions
25 points!!! For the following equation, give the number you would use in the mole-to-mole ratio for each of the reactants and products as well as the number you would use in the volume-to-volume
ratio for each of the gaseous reactants and products. Check to make sure the equation is balanced.
CH4(g) + O2(g) → CO2(g) + H2O (g)
Reactant/Product
CH₂(g)
O₂ (g)
CO₂(g)
H₂O(g)
Mole-to-Mole Number
Volume-to-Volume Number
If you know the volume of substance A, how would you solve for the volume of substance B? Please list the steps you would take in the
correct order.

Answers
For the given equation, the mole-to-mole ratio and volume-to-volume ratio for all the gaseous reactants and products are 1:1.
For the given equation:
CH4(g) + O2(g) → CO2(g) + H2O(g)
Reactant/Product:
CH4(g)
O2(g)
CO2(g)
H2O(g)
Mole-to-Mole Ratio:
By comparing the reactant and product coefficients, we may extract the mole-to-mole ratio from the balanced equation.
CH4(g): 1 mole
O2(g): 1 mole
CO2(g): 1 mole
H2O(g): 1 mole
All of the reactants and products have a mole-to-mole ratio of 1:1:1:1.
Ratio of Volume to Volume:
The ideal gas law, which states that under constant temperature and pressure, the volume of a gas is directly proportional to the number of moles in the gas, can be used to calculate the volume-to-volume ratio.
All gaseous reactants and products have a mole-to-mole ratio of 1:1, hence the volume-to-volume ratio will also be 1:1. As a result, the volume of drug A and substance B will be equal.
You can just take the volume of substance A as the value for substance B if you know the volume of substance A and are trying to solve for the volume of substance B. Alternatively, the quantity of substances A and Bwill be the same, given the 1:1 volume-to-volume ratio.
For more such questions on reactants visit:
https://brainly.com/question/26283409
#SPJ8
How many moles of propane gas would be present in 11 grams
of the gas at standard conditions?
Answers
M
, while the mass is referred to as
m
. The amount of substance is
n
. This gives you the following relationship:
=
M
=
m
n
Since you have given (C3H8)=11 g
m
(
C
3
H
8
)
=
11
g
and you already looked up (C3H8)=44.1 gmol−1
M
(
C
3
H
8
)
=
44.1
g
m
o
l
−
1
, you can use this formula to determine (C3H8)
n
(
C
3
H
8
)
.
In this question it is quite hard to explain the use of significant figures. Those are used to imply a certain inaccuracy. Not enough information is given by the question, as of how accurate the measurement is. It is a mere exercise of converting one property into another. Here you should not worry about it.
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 0.24moles of propane gas would be present in 11 grams of the gas at standard conditions.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
mole of substance =given mass of substance ÷ molar mass of substance
Molar mass of 1 mole of propane gas= 44.0956 g/mol
Given mass of propane gas=11 grams
Substituting all the given values in the above equation, we get
mole of substance =11 grams ÷44.0956 g/mol
mole of substance =0.24moles
Therefore, 0.24moles of propane gas would be present in 11 grams
of the gas at standard conditions.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
A silver bracelet was lowered into a graduated cylinder holding a volume of water equal to 10 mL. The height of the water rose to 14 ml . If the mass of the bracket was 42 g,what is the destiny of silver?
Answers
Answer:
2g
Explanation:
Answer:
\(\boxed {\tt 10.5 \ g/mL}\)
Explanation:
Density can be found by dividing the mass by the volume.
\(d=\frac{m}{v}\)
The mass is 42 grams.
Let's find the volume. The volume was found through water displacement, so we must subtract the final volume of the water with the bracelet (14 mL) and the starting volume of water (10 mL)
v= 14 mL-10 mL v= 4 mLNow we know that:
\(m= 42 \ g\\v= 4 \ mL\)
Substitute the values into the formula.
\(d=\frac{42 \ g }{4 \ mL}\)
Divide.
\(d= 10.5 \ g/mL\)
The density of the silver is 10.5 grams per milliliter.
Penelope's friend Joe gives her a ring for Valentine's Day, which he said is 14k white gold. Penelope's friend Daksha thinks it's actually composed of nickel. Describe a process/experiment that Penelope could conduct to find out for sure.
Answers
Dimethylglyoxime is a chemical that is present in this kit (in solution). KIT The solution will turn pink when used to clean a metal object with such a cotton swab when nickel is present in the Penelope's 14k white gold ring.
Check for the presence of nickel in white gold:White gold was first created to mimic platinum (a white metal). White gold is typically an alloy containing around 75% gold or around 25% nickel and zinc. It would be approximately 75% pure gold if it had an 18 karat stamp.
The question:
Joe, a friend of Penelope's, presents her with a 14k white gold ring on Valentine's Day. Daksha, a friend of Penelope's, believes it is truly made of nickel. Penelope may use the following procedure or experiment to determine this for sure:
You can get a nickel test kit if you're buying jewelry and are unsure if it includes nickel. Dimethylglyoxime is a chemical that is present in this kit (in solution). This chemical is used without harming the thing being evaluated.The solution turns pink when used to clean a metal object with such a cotton swab when nickel is present. This test may not detect trace levels of nickel, but it is typically a reliable jewelry detector.Know more about white gold
https://brainly.com/question/4348678
#SPJ1
why are Van der Waals forces important?
A. they are the strongest type of intermolecular force that can act on polar and non polar molecules.
B. they are the strongest type of intramolecular force that can act between atoms
C. they determine how strong dipole moments will be in polar covalent molecules
D. They are primary intermolecular attractive forces that act between nonpolar molecules
Answers
Answer:
The answer is B.
Explanation:
They are the strongest type of inter-molecular force attractive forces that can act between atoms. This is why Vander Walls forces important.
Van der Waals forces determine how strong dipole moments will be in polar covalent molecules. Therefore, option (C) is correct.
What are van der waals forces?Van der Waals forces are the weakest intermolecular forces that depend on the distance between molecules or atoms. These forces produce from the interactions between non-charged atoms.
Van der Waals forces can form from the disturbance in the polarizations of two atoms when they are close to each other. They fall under the category of ‘weak chemical forces’, as Van der Waals forces are the weakest intermolecular forces.
They vanish when the intermolecular or interatomic distance between the interacting molecules increases. The strengths of Van der Waals forces lie in the range from 0.4 kJ/mol to 4 kJ/mol.
When the electron density of an atom undergoes a transient shift, arises Van der Waals forces. These forces are dependent on the distance between the atoms.
Learn more about Van der Waals forces, here:
https://brainly.com/question/13201335
#SPJ2
* Required
3rd Law Questions
1 point
If a large man pushes a toddler across the ice then which of the following is *
true: (choose all that apply)
a. The man exerts a greater force on the toddler then the toddler does on the man.
b. The man and the toddler push on each other with equal force
c. The man will move a little bit but the toddler will accelerate quickly
d. The toddler has less mass so will have a slower acceleration
what is the answer?
Answers
When we push against an item, the object pushes back against us with an equal and opposite force, according to the third law of motion. if the item is a large truck.
What is an illustration of the third law?According to the third law of motion, there is an equal and opposite reaction to every action. Both objects at rest and those moving faster can exhibit this.
The third law of what?According to his third law, there is an equal and opposite reaction to every force (activity) in nature. If object A applies a force to object B, object B will respond with an equal and opposing force.
To know more about third law of motion visit :-
https://brainly.com/question/29768600
#SPJ1
What is the concentration of H+ in solution given the [OH] = 1.32 x 10^-4? A) 1.0 x 10^14 M B) 7.58 x 10^-11 M C) 1.32 x 10^-11 M D) not enough information E) none of the above
Answers
Option B) 7.58 x 10⁻¹¹ M is the concentration of H+ in solution given the [OH] = 1.32 x 10⁻⁴ will be 1.32 x 10⁻¹¹ M.
We can use the fact that the product of the concentration of hydrogen ions (H⁺) and hydroxide ions (OH⁻) in a solution is equal to 1 x 10⁻¹⁴ M² at 25°C. This is known as the ion product constant of water (Kw).
Mathematically, we can write:
Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴ M²
We are given the concentration of hydroxide ions as [OH⁻] = 1.32 x 10⁻⁴ M. We can use this information and the Kw equation to calculate the concentration of hydrogen ions:
[H⁺] = Kw / [OH⁻]
[H⁺] = (1 x 10⁻¹⁴ M²) / (1.32 x 10⁻⁴ M)
[H⁺] = 7.58 x 10⁻¹¹ M
Therefore, the concentration of H⁺ in solution is 7.58 x 10⁻¹¹ M, which is option B.
learn more about hydrogen here:
https://brainly.com/question/20309096
#SPJ11
7. Cigarette smoke and UV radiation are two
examples of a
Answers
Answer:
carcinogen
Explanation:
cigarette smoke and UV radiation both of the capability to cause cancer cells in living tissue, meaning they can both be classed as carcinogens
please also write out how the hydronium and hydroxide concentrations were calculated for solution 4 in part b.
Answers
In solution 4 of part b, both the hydronium concentration and hydroxide concentration are 1 M due to the neutralization reaction between HCl and NaOH. The initial concentrations of H₃O⁺ and OH⁻ were 0.1 M, but they reacted to form water and resulted in equal final concentrations of 1 M for both ions.
To calculate the hydronium and hydroxide concentrations for solution 4 in part b, we need to first understand the equation for the reaction that occurred.
The equation given is: HCl + NaOH -> NaCl + H₂O
This tells us that one mole of HCl reacts with one mole of NaOH to produce one mole of NaCl and one mole of water.
Based on this equation, we know that the initial concentration of hydroxide ions (OH-) in solution 4 is equal to the initial concentration of sodium hydroxide (NaOH), which was given as 0.1 M.
Next, we need to determine the concentration of hydronium ions (H₃O⁺). Since HCl is a strong acid, it completely dissociates in water to produce H₃O⁺ and Cl⁻ ions. Therefore, we can assume that the initial concentration of H₃O⁺ is equal to the initial concentration of HCl, which was also given as 0.1 M.
Now, let's consider what happens when the HCl and NaOH are mixed together. They react to form NaCl and water, which means that the concentrations of H₃O⁺ and OH⁻ will change.
From the equation, we can see that the reaction consumes one mole of HCl and one mole of NaOH. This means that the final concentration of HCl and NaOH will both be zero.
To determine the final concentration of OH-, we need to use the fact that the reaction produces one mole of water for every mole of NaOH that reacts. Therefore, the final concentration of OH⁻ will be equal to the initial concentration of NaOH (0.1 M) divided by the volume of the solution.
If we assume that the volume of the solution is 100 mL (as stated in the question), then the final concentration of OH- will be:
[OH⁻] = 0.1 M / 0.1 L = 1 M
Finally, we can use the fact that the concentration of H₃O⁺ and OH⁻ must be equal in a neutral solution to determine the final concentration of H₃O⁺.
Since the final concentration of OH⁻ is 1 M, we know that the final concentration of H₃O⁺ must also be 1 M.
To know more about the hydronium & hydroxide concentrations refer here :
https://brainly.com/question/29286376#
#SPJ11
Our atmosphere is made up of 78% N2, 21% O2, and 1% other gases. What is the partial pressure of N2 when atmospheric pressure is 0.980 atm?

Answers
Answer: 0.7644 atm
Explanation:
Given,
Total atmospheric pressure= 0.980atm
Percentage of N₂=78%=0.78
Partial Pressure of N₂=Total atmospheric pressure*Percentage of N₂
=0.980 atm × 0.78
=0.7644 atm
What is the binding energy of 1 mole of 160/50Pm if the mass defect is
0.001328 kg/mol?
I
Answers
Binding energy = 1.1952 x 10 ∧ 14 J/mol
Binding energy - is the amount of energy needed to disperse all of the particles in a system or to remove a particle from it. Subatomic particles in atomic nuclei, electrons attached to atom's nuclei, and atoms and ions bonded together in crystals are three examples of where binding energy is very relevant.
The term "mass defect" refers to the discrepancy between the actual atomic mass and the expected mass obtained by multiplying the mass of the protons and neutrons in the nucleus by a constant factor. The anticipated mass obtained by combining the masses of the nucleons is less than the actual atomic mass.
Given : Mass defect = 0.001328 kg/mol
Speed of light = 3 x 10 ∧8 m/s
Binding energy = Mass defect x speed²
Binding energy = 0.001328 x ( 3 x 10 ∧8 )²
Binding energy = 0.011952 x 10 ∧16
or 1.1952 x 10 ∧ 14
Thus binding energy is 1.1952 x 10 ∧ 14 J/mol
To learn more about mass defect refer- https://brainly.com/question/16485729
#SPJ9
Identify the two gases jn the unknown mixtures.

Answers
Answer:
I believe Gases A and D
Explanation:
The lines in both gases match up with the lines in the unknown mixture.
Answer:
Fa (rt) and Nitrogen
Explanation:
What should the IUPAC name for a binary covalent compound lack? prefixes Roman numerals an -ide ending the name of a nonmetal.
Answers
The IUPAC name for a binary covalent compound will lack roman numerals.
Binary covalent compound comprises of two elements which forms a
compound through the sharing of electrons.
The sharing of electrons is referred to as covalent bonding and naming such
compounds require prefixes such as mono-, di- , tri- etc.
The standard method of naming these compounds should be with the
prefixes and not with the roman numerals.
An example is carbon(IV)oxide which is wrong in IUPAC naming.
Read more on https://brainly.com/question/16731560
Answer:
B
Explanation:
Just did the test on edge 2021
The lead pipe has a mass of 200 g, how much will be left after 6 months?
Answers
Answer:
33g
Explanation:
200 divided by 6=33.33
2C2H6 + 7O2 → __CO2 + 6H2O
What coefficient will need to be placed in the blank in order for the following equation to be correctly balanced?
A.
1
B.
3
C.
4
D.
7
Answers
Answer:
From the Combustion formula of Organic Compounds....
Exactly 4 Moles of CO2 would make the equation balanced
So
OPTION C
4MOLES
Which group of coefficients balances the following chemical equation? 2KCIO3 → _KCI + _O2 a 1, 3 b 2,2 C 2,3 d 2.1 e 1, 1
Answers
Answer:
2KClO3 = 2KCl + 3O2
I hope it's helps you
Someone plz help I Don’t know I would do something like this and I really need to get it done
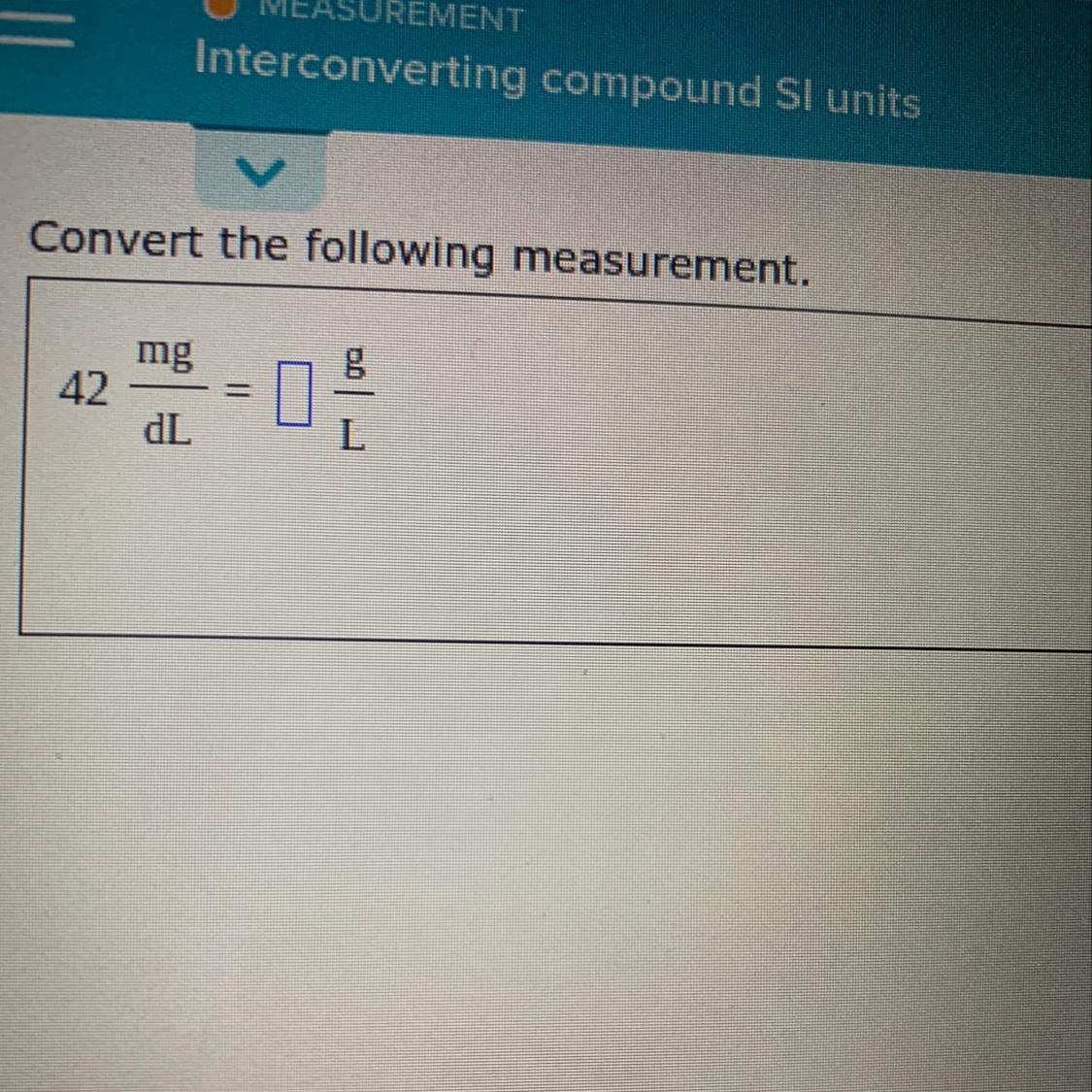
Answers
1dL =.1L
0.001/.1 *42=0.42
Some bacteria live in the roots of plants like soybeans and peas.
Bacteria growing on plant roots.
What is the role of these bacteria in the nitrogen cycle?
to absorb nitrogen-containing compounds from the soil
to release free nitrogen into the atmosphere
to break down nitrogen-containing compounds in dead organisms
to convert free nitrogen into usable nitrogen
70 points!
Answers
Answer:
to release free nitrogen into the atmosphere. This is the answer.
Answer:
to convert free nitrogen into usable nitrogen
Explanation:
got it right on the test boi
when HCl react with finely powdered iron it form ferrous chloride and not ferric chloride. why?
Answers
Answer:
When iron is allowed to react with HCl,
It produces hydrogen and the release of hydrogen gas prevents the formation of ferric chloride.
Hope it helps
Thank you
Please mark me as brainliest.
How many neutrons are in atom of an element that has 16 protons, 16 electrons, and a mass of 40?
Answers
Answer:
8
Explanation:
i dunno i think its right tho
What is produced at the anode in the electrolysis of molten CuF2? O A. F2 O B. H2 O C. cu D.02
Answers
The produced at the anode in the electrolysis of molten CuF2 is A. F₂.
During electrolysis, CuF₂ will separate into Cu₂⁺ cations and F⁻anions. Cu₂⁺ will be reduced to copper metal, which will deposit on the cathode, whereas F⁻ anions will be oxidized to fluorine gas on the anode in the electrolysis of molten CuF₂. Hence, the answer is option A. Fluorine gas (F₂) is generated at the anode in the electrolysis of molten CuF₂.
Therefore, during the electrolysis of molten CuF₂, Cu₂⁺ is reduced to copper metal, which deposits on the cathode, and F⁻ anions are oxidized to fluorine gas on the anode, which is produced at the anode. The chemical reactions taking place during electrolysis of CuF₂ are given below: At the cathode, Cu₂⁺ cations get reduced to copper metal. Cu₂⁺ + 2e⁻ ⟶ Cu. At the anode, F⁻ anions get oxidized to fluorine gas. 2F⁻ ⟶ F₂ + 2e⁻. Therefore, option A is correct.
Learn more about electrolysis at:
https://brainly.com/question/12994141
#SPJ11
Fe + AgNO3 → Fe(NO3)2 + Ag
How much iron is needed to make 7.2mol Iron (II) Nitrate?
Answers
3.6 moles of iron are needed to produce 7.2 mol of Iron (II) Nitrate.
To determine the amount of iron needed to produce 7.2 mol of Iron (II) Nitrate (Fe(NO3)2), we need to use the stoichiometry of the balanced chemical equation.
From the balanced equation:
Fe + 2AgNO3 → Fe(NO3)2 + 2Ag
We can see that one mole of iron reacts with two moles of silver nitrate to produce one mole of Iron (II) Nitrate and two moles of silver.
To find the amount of iron needed, we will set up a proportion based on the stoichiometric ratio:
1 mol Fe / 2 mol AgNO3 = x mol Fe / 7.2 mol Fe(NO3)2
Cross-multiplying and solving for x, we get:
x = (1 mol Fe / 2 mol AgNO3) * 7.2 mol Fe(NO3)2
x = 3.6 mol Fe
Therefore, 3.6 moles of iron are needed to produce 7.2 mol of Iron (II) Nitrate.
It is essential to note that in stoichiometry calculations, the balanced chemical equation is used to establish the molar ratios between reactants and products. These ratios allow us to calculate the amounts of substances involved in a chemical reaction.
By applying the stoichiometry concept, we can determine the appropriate amount of reactants required to produce a desired amount of product, as demonstrated in this case where we calculated the necessary moles of iron to produce 7.2 mol of Iron (II) Nitrate.
Know more about balanced chemical equation here:
https://brainly.com/question/26694427
#SPJ8
Weathering notes
The breaking down of ________ into smaller_________ next to each other. Weathering forms ____________
Mechanical weather.
_____________ breaking of rock without any ____________ in the _____________ composition of the rock
Ice ___________
_______________
_______________
_____________.
(Force)
Chemical weathering
The breaking down of rock into smaller _____________ because of __________ changes within the rock
______________
_____________
__________________
_____________
(Acid)
Answers
Answer:
i have no idea this is to hard for me ;(4
Explanation:
when 39.8 g of a nondissociating, nonvolatile sugar is dissolved in 200.0 g of water, the boiling point of the water is raised by 0.30°c. estimate the molar mass of the sugar.
Answers
The molar mass of the nondissociating, nonvolatile sugar is estimated to be 342.73 g/mol.
When 39.8 g of a nondissociating, nonvolatile sugar is dissolved in 200.0 g of water, the boiling point of the water is raised by 0.30°C. To estimate the molar mass of the sugar, we can use the formula:
∆Tb = (Kb x W2)/W1
Here, Kb is the boiling point elevation constant with a value of 0.512 °C/m. W1 represents the mass of the solvent in kilograms, which is 0.200 kg. W2 is the mass of the solute in kilograms.
Given that the boiling point elevation (∆Tb) is 0.30°C and the mass of the solute (sugar) W2 is 39.8 g (0.0398 kg), we can calculate the molar mass using the formula:
Molar mass = (mass × (R × ∆Tb)) / (Kb × W2)
Substituting the known values:
Molar mass = (0.0398 × (8.31 × 0.30)) / (0.512 × 0.200)
After calculation, we find that the molar mass of the nondissociating, nonvolatile sugar is estimated to be 342.73 g/mol.
To know more about topic Colligative properties and Molar mass here: https://brainly.com/question/32797671
#SPJ11
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
What is the percent, by mass, of water in MgSO4.2H20
Answers
Answer:51.1%
Explanation:
Mass percent : It is defined as the mass of the given component present in the total mass of the compound. Formula used : First we have to calculate the mass of and . Mass of = 18 g/mole Mass of = 7 × 18 g/mole = 126 g/mole Mass of = 246.47 g/mole Now put all the given values in the above formula, we get the mass percent of in . Therefore, the mass percent of in is, 51.1%
To which galaxy does the Sun belong?
A Centaurus
B Milky Way
C Andromeda
D Canis Major
Pls help
Answers
Graph the pH function using the graphing utility. Then answer the questions. What is the pH of lemon juice that has a hydrogen ion concentration of 0. 01 moles per liter? The pH is.
Answers
The pH of lemon juice has a hydrogen ion concentration of 0. 01 moles per liter is 2.
What is pH?pH is the measurement of acidity and basicity of any solution. The meaning of pH is potential hydrogen.
The acidity or basicity of any solution can be measured by pH scale, which is from 0 to 14.
The pH for neutral is 7 which is of pure water
The pH above 7 is base and pH below 7 is acids.
Thus, the pH of Lemon juice is 2.
Learn more about pH, here:
https://brainly.com/question/491373
How many moles of gas are in a gas sample occupying 5.6 L at 690 mmHg and 20°C?
Select one:
a. 0.211 moles
b. 161 moles
c. 23.4 moles
d. 2.74 moles
Answers
To solve this problem, we need to use the Ideal Gas Law equation. The answer is option a) 0.211 moles.
To solve this problem, we need to use the Ideal Gas Law equation which is PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, and T is the temperature in Kelvin.
First, we need to convert the given temperature of 20°C to Kelvin by adding 273 to it. So, T = 293 K.
Next, we need to convert the given pressure of 690 mmHg to atm by dividing it by 760 mmHg/atm. So, P = 0.908 atm. Now, we can rearrange the Ideal Gas Law equation to solve for n:
n = (PV)/(RT)
Substituting the given values, we get:
n = (0.908 atm * 5.6 L)/(0.0821 L*atm/mol*K * 293 K)
Simplifying this expression, we get:
n = 0.211 moles
For more questions on Gas Law equation
https://brainly.com/question/4147359
#SPJ11
the term pertaining to all the chemical reactions and physical workings of the cell is
Answers
Metabolism is referred as the term pertaining to all the chemical reactions and physical workings of the cell.
Cellular metabolism refers to the set of chemical and physical processes that occur within a cell in order to convert nutrients into energy and other molecules that the cell can use. It also includes the transport of molecules in and out of the cell, as well as the regulation of these processes which helps to maintain the homeostatic balance of the cell, allowing it to function normally and respond to environmental changes. This includes the breakdown of molecules like carbohydrates, proteins, and lipids into their basic components, as well as the synthesis of molecules like enzymes, hormones, and other complex molecules.
To learn more about metabolism click here https://brainly.com/question/29523568
#SPJ4