What is the molecular mass of Br 2?
Answers
Answer:
159.808 g/mol
Answer:
159.80 g/mol
Explanation:
79.90×2=159.80g/mol
Related Questions
After a 4.626g sample of silver oxide is heated, 4.306g of silver metal remains. What is the empirical formula of the compound?
please help me ;(
Answers
Answer:
The empirical formula is Ag2O.
The empirical formula is Ag2O.Explanation:
The empirical formula is Ag2O.Explanation:The empirical formula is the simplest whole-number ratio of atoms in a compound.
The empirical formula is Ag2O.Explanation:The empirical formula is the simplest whole-number ratio of atoms in a compound.The ratio of atoms is the same as the ratio of moles. So our job is to calculate the molar ratio of Ag to 2O.
do the steps ...
To get this into an integer ratio, we divide both numbers by the smaller value.
From this point on, I like to summarize the calculations in a table.
ElementAgMass/gXMolesXllRatiomllIntegers
—————————————————−———mAgXXXm7.96Xm0.07377Xll2.00mmm2
mlOXXXXl0.59mm0.0369Xml1mmmml1
There are 2 mol of Ag for 1 mol of O.
Answer:
Hello There!!
Explanation:
The answer is↬The empirical formula is Ag2O. It is the simplest whole-number ratio of atoms in a compound.
hope this helps,have a great day!!
~Pinky~
The recipe for an individual pizza is 1 crust, 1 scoop of tomato sauce, 0.5 cups of shredded mozzarella cheese, and 9 slices of pepperoni. In your kitchen, you have 2 crusts, 4 scoops of tomato sauce, 1 cup of cheese, and 9 slices of pepperoni. Which ingredient is the excess reactant?
A. pepperoni
B. tomato sauce
C. crust
D. cheese
Answers
The excess reactant in the preparation of pizza from the given materials is tomato sauce; option B
What is an excess reactant?In a chemical reaction, the excess reactant is the reactant that is left over at the end of the reaction.
The excess reactant is present in a quantity greater than the mole ratio of the reactant to form a product.
Considering the given reaction and the mole ratio of the reaction:
Recipe for 1 pizza = 1 crust, 1 scoop of tomato sauce, 0.5 cups of shredded mozzarella cheese, and 9 slices of pepperoni.
Amount present = 2 crusts, 4 scoops of tomato sauce, 1 cup of cheese, and 9 slices of pepperoni.
Excess reactant = tomato sauce.
Learn more about excess reactants at: https://brainly.com/question/25685654
#SPJ1
Answer:
Cheese
Explanation:
Did the lab
What must occur for a hypothesis to be considered fully proven?
A
Newspapers must report the event.
B
Public opinion must agree with the results.
C
Other scientists must repeat the experiment and get the same results.
D
There must be a specific number of controlled experiments.
Answers
IM GIVING BRAINLEST!
What is the name of Pb(NO3 ) 2 Explain how you determined the bond type and the steps you used to determine the naming convention for the compound
Answers
Name of Pb(NO3 )2 is Lead(II) nitrate.
Pb(NO3)2 is ionic as well as covalent
Pb is the symbol of lead and NO3 is a complex ion called nitrate. It has one nitrogen and three oxygens. Pb(NO3)2 is ionic as well as covalent. NO3 is covalent in nature because both nitrogen and oxygen are non-metals and therefore an ionic bond cannot be formed between them.
Ionic compounds are formed when a metal reacts with a nonmetal whereas covalent compounds are formed when two non -metals react with each other. Binary compounds containing hydrogen are usually covalent compounds as hydrogen is a non - metal.
An ionic bond donates an electron to the other atom in the bond whereas electrons in a covalent bond are shared equally between the atoms.
To know more about bond types, refer
https://brainly.com/question/2234173
#SPJ13
A man needs to lift a heavy box on the back of a truck or push up a ramp or lift it straight up from the ground to use around he won't have to move the box over a longer distance what did is the benefit of using a ramp to get the box
Answers
Do we need to worry about Earth's heat running out? Why or why not?
Answers
While that sounds frightening, some estimates place the cooling of the Earth's core at tens of billions of years, or as much as 91 billion years. That is a very long time, and the Sun will most likely burn out much sooner than the core in around 5 billion years.
Why do you think the heating of the Earth is important?This heat is responsible for plate tectonics and parts of the rock cycle. The geological processes that transform the three major rock types (igneous, metamorphic, and sedimentary) from one to the other.
The internal heat of the Earth is critical for processes that result in the transformation of one rock type to another.
Thus, yes, we need to worry about Earth's heat running out
To learn more about earth, follow the link;
https://brainly.com/question/12041467
#SPJ1
a compound was analyzed and found to contain 13.5 grams calcium, 10.8 grams oxygen, and 0.675 grams of hydrogen. what is the empirical formula for the compound?
Answers
The empirical formula for the compound is Ca(OH)₂.
To determine the empirical formula of the compound, we need to find the simplest whole-number ratio of the elements present. We can do this by calculating the number of moles of each element and then finding the ratio between them.
Mass of calcium (Ca) = 13.5 grams
Mass of oxygen (O) = 10.8 grams
Mass of hydrogen (H) = 0.675 grams
First, we need to convert the masses of each element to moles using their respective atomic masses.
Atomic mass of Ca = 40.08 g/mol
Atomic mass of O = 16.00 g/mol
Atomic mass of H = 1.01 g/mol
Number of moles of Ca = 13.5 g / 40.08 g/mol = 0.3367 mol
Number of moles of O = 10.8 g / 16.00 g/mol = 0.675 mol
Number of moles of H = 0.675 g / 1.01 g/mol = 0.6693 mol
Next, we need to find the simplest whole-number ratio by dividing the number of moles of each element by the smallest number of moles obtained.
Dividing by 0.3367 (smallest value):
Number of moles of Ca = 0.3367 mol / 0.3367 mol = 1
Number of moles of O = 0.675 mol / 0.3367 mol = 2.005
Number of moles of H = 0.6693 mol / 0.3367 mol = 1.988
Rounding these values to the nearest whole number, we have:
Number of moles of Ca = 1
Number of moles of O = 2
Number of moles of H = 2
Thus, the empirical formula of the compound is Ca(OH)₂.
Learn more about empirical formulas at https://brainly.com/question/13058832
#SPJ11
which procedure is recommended when a student needs more of a hazardous material
Answers
When a student needs more of a hazardous material, the recommended procedure is to follow proper safety protocols and guidelines, including consulting with the instructor or supervisor.
Handling hazardous materials requires careful consideration of safety measures to minimize the risks involved. In this situation, the student should first consult with their instructor or supervisor to communicate their need for more of the hazardous material. The instructor or supervisor can provide guidance on the appropriate procedures to follow, including assessing the necessity for the material and ensuring that the student has the necessary training and knowledge to handle it safely.
It is crucial to understand the risks associated with the hazardous material and follow all safety guidelines and protocols. This may include wearing appropriate personal protective equipment (PPE), working in a well-ventilated area, and using proper storage and disposal methods.
Furthermore, it is important to adhere to any legal requirements or regulations regarding the procurement and handling of hazardous materials. This may involve obtaining the necessary permits or licenses, ensuring compliance with safety standards, and maintaining proper documentation.
By following these recommended procedures, students can ensure their safety and the safety of others while obtaining the required amount of hazardous materials.
Learn more about hazardous material here: brainly.com/question/30264140
#SPJ11
how many moles are in 37.5 g nitrogen
Answers
Aluminum foil, cast iron pans, copper pipes, graphite, and sea water can be classified as
A) insulators because free electron flow is hindered by the structure of the material.
Eliminate
B) conductors because electrons can move freely from one atom to another creating a current.
C) superconductors because they provide no resistance to electron flow through the material.
D) semiconductors because electron movement is dependent on the conditions - such as temperature and voltage.
Answers
answer
C) is the answer ⟵(๑¯◡¯๑)
A model that describes the formation, ______________, and reformation of rocks.
Answers
Change 1
Change 1 is:
impossible
a physical change
a chemical change
Sample of Substance X
Change 2
Change 2 is:
impossible
a physical change
a chemical change
Change 3
Change 3 is:
impossible
a physical change.
a chemical change
X
S
Answers
In the original sample of substance X, there are 12 molecules and each molecule is made of 2 small red spheres and one blue sphere in the middle.
Change 1:Change 1 has the same total number of red and blue spheres as the original sample, but the structure of molecules has changed. As a result, this is a chemical change.
Change 2:In Change 2, the structure of molecules is changed. Some molecules contain solely red spheres, whereas others include both red and blue spheres. Furthermore, the total number of molecules is greater than the number of molecules in the original sample. This violates the principle of conservation of mass. Hence, this is an impossible change since extra matter was created in this change.
Change 3:In Change 3, the number of the molecules and the structure of the molecules is same as the orignial sample. Hence, it is a physical change.
Read more about Physical and Chemical Changes:
https://brainly.com/question/4159679
#SPJ4

What kind of food provides energy for much of the human population
Answers
The food substance which gives energy for humans are the carbohydrates.
Why carbohydrates provides energy?It follows that among the classes of food substances, the group of food or diet which has the highest calories supply of energy are the carbohydrates. These carbohydrates are the energy giving food.
Aside carbohydrates, other food classes include the following:
ProteinsMineral saltsVitaminsFats and oilsRoughagesWater.The proteins helps in building the body. The vitamins are the body regulating and protecting food.
So therefore, we can now deduce that when we consume foods such as rice, maize and others forms of carbohydrates, it gives us energy.
Read more on carbohydrates:
https://brainly.com/question/797978
#SPJ1
Which is the correct equation for the reaction of magnesium with hydrochloric acid to produce hydrogen gas and magnesium chloride?
O Mg + 2HCl -> 2H + MgCl2
O Mg + 2HCl -> H2 + MgCl2
O2Mg + 6HCl -> 3H2 + 2MgCl2
O Mg + HCl -> H+MgCl
Answers
Answer:
Mg(s) + 2 HCl(aq) --> MgCl 2(aq) + H 2(g)
Propose a structure for compounds consistent with the following mass spectral data: (a) A ketone with M+=86 and fragments at m/z=71 and m/z=43 (b) An alcohol withi M+=88 and fragments at m/z=73, m/z=70, and m/z=59 (c) A hydrocarbon with M+=84
Answers
The ketone that can be shown by the description have been shown in the image attached.
What does mass spectroscopy show us?A strong analytical method known as mass spectrometry can reveal important details about the make-up, structure, and characteristics of molecules. It enables researchers to pinpoint and examine a sample's chemical and isotopic features.
The mass of a molecule or an ion can be precisely determined using mass spectrometry. The mass-to-charge ratio (m/z) measurement enables the recognition and verification of a compound's molecular formula. Particularly relevant to the study of pharmaceutical analysis, biochemistry, and organic chemistry.
Learn more about spectroscopy:https://brainly.com/question/31594990
#SPJ4

A new element named antmanium is added to the periodic chart. The number written below the symbol is 444.41. There are four isotopes with one being 90% abundant. What is the name of the most abundant isotope
Answers
The name of the most abundant isotope of antmanium is not provided in the question. Therefore, the most abundant isotope would have an atomic mass close to the given number, which is 444.
The number written below the symbol of antmanium (444.41) is likely its atomic mass, which is the sum of the masses of all its isotopes. However, the information about the isotopes provided in the question does not include the names or masses of the individual isotopes, only that there are four isotopes and one is 90% abundant. Without knowing the individual masses or names of the isotopes, we cannot determine which is the most abundant.
To name isotopes, we combine the element's name with its atomic mass number (the sum of protons and neutrons in the nucleus). In this case, the element is antmanium, and the atomic mass number is 444, so the isotope's name is Antmanium-444.
To know more about atomic mass visit:
https://brainly.com/question/30050618
#SPJ11
Which sentence most accurately describes the daily growth rate of plants? (1 point)
Answers
Answer: D- average change in plant height, divided by, the number of days.
(i had to look up the test you were talking about to find what the answer choices were.)
Hope this helps!
a mixture of 0.220 moles rn, 0.350 moles o2 and 0.640 moles ar has a total pressure of 1.65 atm. what is the partial pressure in atm of o2?
Answers
The partial pressure in atm of O2 is 0.47 atm
What do you mean by moles?
A mole is simply a unit of measurement. In fact, it's one of the seven base units in the International System of Units (SI). Units are invented when existing units are inadequate. Chemical reactions often take place at levels where using grams wouldn't make sense, yet using absolute numbers of atoms/molecules/ions would be confusing, too. So, scientists invented the mole to bridge the gap between very small and very large numbers.
No. of moles of Rn = 0.220 moles
No. of moles of O2 = 0.350 moles
No. of moles of Ar = 0.640 moles
Total no. of moles = 1.21 moles
Mole fraction of O2 , xO2 = 0.350moles / 1.21 moles = 0.289
Total pressure = 1.65 atm
Partial Pressure of O2 = Mole fraction of O2* Total Pressure
= 0.289 * 1.65 atm
= 0.47 atm
To know more about moles from the given link:
https://brainly.com/question/21911991
#SPJ4
How can you tell if an atom is an isotope?
Answers
Answer:
Look up at the atom on the periodic table of elements and find out what its atomic mass is. Subtract the number of protons from the atomic mass. This is the number of neutrons that the regular version of the atom has. If the number of neutrons in the given atom is different, than it is an isotope.
can someone help me please
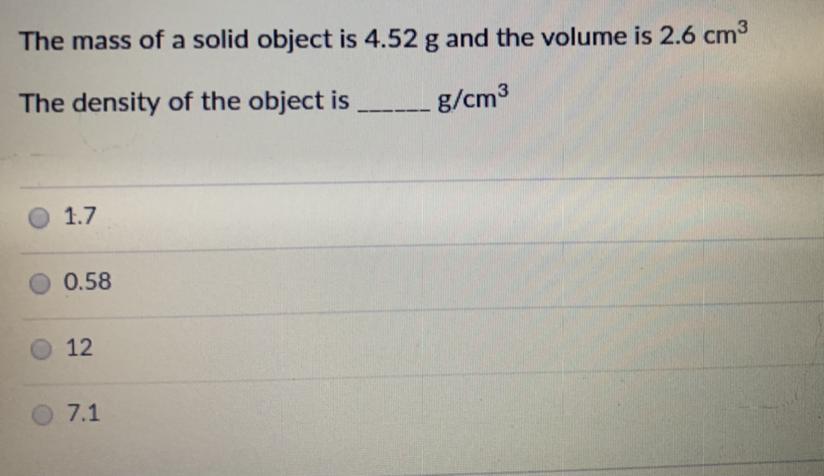
Answers
chlorine is an active non metal
Answers
Chlorine in group 17 in the periodic table and includes halogens (also fluorine, bromine, and iodine)
Electron configuration of Cl : [Ne] 3s² 3p⁵
From this configuration, it can be seen that to obtain stability like the noble gas Ar - [Ne] 3s² 3p⁶, Chlorine will need one more electron so that its configuration is the same as Ar( become anion-Cl⁻)
Then it is said that Cl is reactive
And the Cl element is included in the non-metal group because it attracts electrons (electronegative)
The elements that include non-metals are halogens, noble gases, and 7 elements H, C, N, O, P, S, Se.
An object is traveling at a speed of 500.0 m/sec. How fast is that in km/hr?
Answers
Answer:
0.5km/hr
Explanation:
\(500.0 \div 1000\)
this is because to get from n to KM you have to divide by 1000
When we inhale oxygen, we expire it as water vapors. We can regenerate oxygen by reacting the water vapor with potassium superoxide KO2. When we react 25.0 grams of water vapor with 25.0 grams of potassium superoxide, we obtain 15.0 grams of potassium hydroxide. Oxygen is also created during the reaction. What is the theoretical and percent yield of potassium hydroxide?
Answers
The theoretical and percent yield of potassium hydroxide would be 19.771 g and 75.83% respectively.
Stoichiometric problemThe balanced chemical equation for the reaction between water vapor and potassium superoxide is:
4 KO2 + 2 H2O → 4 KOH + 3 O2
From the balanced equation, we can see that for every 4 moles of KO2 reacted, 4 moles of KOH are produced.
Calculate the number of moles of KO2 used:
25.0 g KO2 × (1 mol KO2 / 71.098 g KO2) = 0.3520 mol KO2
Calculate the number of moles of KOH produced:
According to the balanced equation, 4 moles of KO2 produce 4 moles of KOH, so:
0.3520 mol KO2 × (4 mol KOH / 4 mol KO2) = 0.3520 mol KOH
Calculate the theoretical yield of KOH:
The molar mass of KOH is 56.105 g/mol, so:
0.3520 mol KOH × 56.105 g KOH/mol = 19.771 g KOH
Therefore, the theoretical yield of KOH is 19.771 g.
Calculate the percent yield of KOH:
The actual yield of KOH is given as 15.0 g, so:
Percent yield = (actual yield / theoretical yield) × 100%
Percent yield = (15.0 g / 19.771 g) × 100%
Percent yield = 75.83%
Therefore, the percent yield of KOH is 75.83%.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
A 20.0 mL solution of NaOH is neutralized with 24.1 mL of 0.200 M HBr. What is the concentration of the original NaOH solution
Answers
Answer:
0.241 M
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
HBr + NaOH —> NaBr + H₂O
From the balanced equation above,
The mole ratio of acid, HBr (nₐ) = 1
The mole ratio of base, NaOH (n₆) = 1
Finally, we shall determine the concentration of the NaOH solution. This can be obtained as follow:
Volume of base, NaOH (V₆) = 20 mL
Volume of acid, HBr (Vₐ) = 24.1 mL
Concentration of acid, HBr (Cₐ) = 0.2 M
Concentration of base, NaOH (C₆) =?
CₐVₐ / C₆V₆ = nₐ/n₆
0.2 × 24.1 / C₆ × 20 = 1/1
4.82 / C₆ × 20 = 1
Cross multiply
C₆ × 20 = 4.82
Divide both side by 20
C₆ = 4.82 / 20
C₆ = 0.241 M
Therefore, the concentration of the NaOH solution is 0.241 M
which gas is not an example of a naturally occurring greenhouse gas?responsesnitrous oxidenitrous oxidemethanemethanechlorofluorocarbonchlorofluorocarbonwater vapor
Answers
Chlorofluorocarbons (CFCs) are not naturally occurring greenhouse gases.
Chlorofluorocarbons (CFCs) are not naturally occurring greenhouse gases. They are synthetic compounds that were widely used in refrigerants, solvents, and aerosol propellants.
CFCs were found to deplete the ozone layer in the upper atmosphere, leading to the adoption of the Montreal Protocol to phase out their use.
While they are not naturally occurring, they contribute significantly to global warming due to their strong greenhouse effect.
To know more about Chlorofluorocarbons refer here:
https://brainly.com/question/16159504
#SPJ11
Pls help!
Many thanks

Answers
The shortest covalent bond is the H-F bond. Therefore, option (A) is correct.
What is the bond length order of hydrogen halides?The electronegativity decreases down the group and the Fluorine is the most electronegative atom in the periodic table. The size of the atoms also increases as we go down the group.
The bond length of the hydrogen halides will follow the order HF < HCl < HBr < HI. The radius of the Iodine atom is the largest and to form a molecule, the hydrogen atom will be farthest as compared to all hydrogen halides.
The molecule with Fluorine will have the shortest bond length because its atom has the smallest size, making them attach very closely. So the covalent bond of hydrogen fluoride will be the shortest.
The relationship between bond length and bond strength is inverse in nature. The bond length of HI is the greatest, it will have the least bond strength. So the order for bond strength of the hydrogen halides is HF > HCl > HBr > HI.
Learn more about hydrogen halides, here:
https://brainly.com/question/12115740
#SPJ1
The speed of light in a vacuum is 2.998×10^8 m/s .
What is its speed in miles per minute (mi/min)?
Answers
Answer:299,800,000
Explain: The speed of light is 3.00x108m/s and in mph is 6.708 x 108.
What is the state of oxygen at -200 degrees celsius
Answers
Answer:
i think its going to be liquid
Explanation:
Discuss how chemical bonding explain the properties of chemical and biological polymers
Answers
Chemical bonding explains the properties of chemical and biological polymers by forming strong covalent bonds or flexible hydrogen bonds, which give the polymer its unique characteristics.
Exploring the Role of Chemical Bonding in the Properties of Chemical and Biological PolymersChemical bonding is a fundamental concept that explains the properties of chemical and biological polymers. Chemical bonds are formed when atoms interact with each other to form molecules or particles. In a polymer, the atoms are linked together in a repeating pattern, forming a long chain. These bonds give the polymer its unique properties, such as strength, flexibility, and the ability to interact with other molecules. The type of chemical bond formed between the atoms will determine the properties of the polymer.
Learn more about chemical bonds: https://brainly.com/question/20387565
#SPJ4
he metric system is now known as the international system of units.
Answers
True, the metric system is now known as the international system of units.
What is the metric unit?A system of measurement in which the basic units are the meter, the second, and the kilogram.
The Metric System is also known as The International System of Units. The metric system is called that because it is the system that the vast majority of countries in the world currently use.
The only countries that do not use this system are Liberia, Myanmar, and the United States, although schools in the United States are working to teach students both variations.
Learn more about the metric unit here:
https://brainly.com/question/401990
#SPJ1