What is the molarity of a solution prepared by dissolving 198 g of BaBr2 in 2.00 liters of solution?
Answers
Answer:
The molarity of the solution is 0.335 \(\frac{moles}{liter}\)
Explanation:
Molar concentration is a measure of the concentration of a solute in a solution, be it some molecular, ionic, or atomic species.
Molarity is the number of moles of solute that are dissolved in a certain volume and is calculated by:
\(Molarity=\frac{number of moles of solute}{volume}\)
Molarity is expressed in units \(\frac{moles}{liter}\).
Being the molar mass of BaBr2 equal to 297.14 g/mole, that is to say that 1 mole contains 297.14 grams, the mass of 198 grams are contained in:
\(198 grams*\frac{1 mole}{297.14 grams} = 0.67 moles\)
So:
number of moles of solute= 0.67 molesvolume= 2 LReplacing in the definition of molarity:
\(Molarity=\frac{0.67 moles}{2 L}\)
Solving:
Molarity= 0.335 \(\frac{moles}{liter}\)
The molarity of the solution is 0.335 \(\frac{moles}{liter}\)
Related Questions
bismuth-210 is an isotope that radioactively decays by about 13% each day, meaning 13% of the remaining bismuth-210 transforms into another atom (polonium-210 in this case) each day. if you begin with 193 mg of bismuth-210, how much remains after 6 days?
Answers
After 6 days, approximately 56.5 mg of bismuth-210 remains.
To learn more about bismuth here:
https://brainly.com/question/13721046
#SPJ11
using values from appendix c, calculate the standard enthalpy change for each of the following reactions: (a) 2 so21g2 o21g2¡ 2 so31g2 (b) mg1oh221s2¡ mgo1s2 h2o1l2 (c) n2o41g2 4 h21g2¡ n21g2 4 h2o1g2 (d) sicl41l2 2 h2o1l2¡ sio21s2 4 hcl1g2
Answers
To calculate the standard enthalpy change for each of the reactions, we need to use the values from Appendix C.
Here are the calculations:
(a) 2 SO2(g) + O2(g) → 2 SO3(g)
ΔH = (2 × ΔHf[SO3]) - (2 × ΔHf[SO2]) - (ΔHf[O2])
(b) Mg(OH)2(s) → MgO(s) + H2O(l)
ΔH = ΔHf[MgO] + ΔHf[H2O] - ΔHf[Mg(OH)2]
(c) N2O4(g) + 4 H2(g) → 2 NH3(g) + 4 H2O(g)
ΔH = (2 × ΔHf[NH3]) + (4 × ΔHf[H2O]) - (ΔHf[N2O4]) - (4 × ΔHf[H2])
(d) SiCl4(l) + 2 H2O(l) → SiO2(s) + 4 HCl(g)
ΔH = ΔHf[SiO2] + (4 × ΔHf[HCl]) - ΔHf[SiCl4] - (2 × ΔHf[H2O])
Remember to substitute the values from Appendix C for ΔHf[...] and perform the calculations. Ensure the units are consistent.
to know more about enthalpy visit:
https://brainly.com/question/16994235
#SPJ11
what is hard water and soft water
Answers
Answer:
Hard water is water that has high mineral content, Soft water is free from dissolved salts of such metals as calcium, iron, or magnesium
Explanation:
Which prediction best shows what the population could look like after many generations? What caused it to change?
Answers
Prediction 1 best shows what the population could look like after many generations. Because, the long beak traits is having more life time.
What is genetic traits ?Each characteristics in a living thing is created by a respective genetic coding in its body. The genetic code which is responsible for a particular behavior or appearance is called a genetic trait.
Here, the prediction 1 is best . It already saying that long-beak hummingbirds are more likely to survive, that baby survived long enough to pass on its mutation, so the long-beak trait became more common over generations.
Prediction 2 says abut a genetic trait which is less fit to survive. Hence, cannot affect the next generation population. Therefore, prediction 1 is best.
Find more on genetic traits:
https://brainly.com/question/29679846
#SPJ1
Your question is incomplete. But your complete question probably was:
Prediction 1 :Two hummingbirds with short or medium beaks had a baby with a mutation in its genes for the long-beak trait.
Prediction 2 is best. A hummingbird could have been born with a mutation in its genes for the long-beak trait and lived for a little while.
4.13 moles of chromium (III) nitride weighs how much?
Answers
Answer: 272.5915639999...
Explanation:
List the celestial bodies from smallest to largest based on their actual size.
Answers
The list of celestial bodies from smallest to largest are asteroids, moons, planets, and Stars.
What are celestial bodies?
A celestial body is an occurrence that naturally takes place in space. The terms object and body can be used interchangeably when referring to space and galaxies. Celestial bodies are firmly bound things with intricate shapes and structures that contain hundreds of smaller items.
The sun, moon, stars, and planets are examples of celestial bodies, sometimes known as heavenly bodies. The cosmos contains celestial objects, which are cosmic bodies that are visible in space and are positioned distant from the earth. We can only see the majority of the celestial groupings with a telescope.
They are usually seen at night and are invisible to the unaided eye. The size of certain celestial bodies is considerably greater than that of the planet. They are located outside of the atmosphere of the earth. There are millions of celestial bodies in the Kuiper belt.
Therefore, the list of celestial bodies, from smallest to largest are asteroids, moons, planets, and stars.
Read more about celestial bodies, here
https://brainly.com/question/2964397
#SPJ2
What should the plan of care for a client who takes lithium include?
Emphasis on monitoring weight gain carefully
Dietary teaching to restrict daily sodium intake
Periodic laboratory monitoring of renal and thyroid function
Importance of discontinuing the medication if fine hand tremors occur
Answers
The plan of care for a client who takes lithium should include periodic laboratory monitoring of renal and thyroid function and the correct option is option 3.
Two major long-term risks of lithium therapy are hypothyroidism and impairment of the kidney's ability to concentrate urine
Therefore, a person receiving lithium therapy must have periodic follow-ups to assess thyroid and renal function.
Weight gain and fine tremors are common side effects associated with this medication, but the patient should continue taking the medication.
Thus the ideal selection is option 3.
Learn more about Lithium intake, here:
https://brainly.com/question/31449457
#SPJ4
Why do different elements emit different colors of light?
Answers
Answer:
Different elements emit differently colors of light because when electrons return to the lower energy levels, they emit energy in a different form of light
Explanation:
Critical Th
Use this phof
following qu
Lesson Beviel
Vocabulary
Circle the term that best completes each of the
following sentences.
1 A(n) hypothesis/observation is tested in
an experiment.
94+ yell.52
2 In an experiment, the independent/dependent
variable is the one that scientists manipulate
on purpose.
Answers
Answer:
1) hypothesis
2. independent .
hypothesis is a guess that is tested by an experiment
independent variable is manipulated by the experimenter
An electron in a hydrogen atom is excited from the n = 1 ground state to the n = 4 excited state. Classify the statements about this absorption and emission process as true or false. False True It takes less energy to contre the electron from the state than from the ground state Os avere, the electron is closer to the miles in the state than in the state The wavelength of light emitted when the electron falls from = 40 2 is shorter than the wavelength of light emitted when the electron falls from = 4 ton. The wavelength of light absorbed when the electron is excited from the ground stetos = 4 is the same as the wavelength of light emitted when the electron falls from 4to the ground te The excited state is the first possible excited state
Answers
True:
It takes more energy to excite the electron from the n = 4 state than from the ground state.
On average, the electron is closer to the nucleus in the n = 1 state than in the n = 4 state.
The wavelength of light emitted when the electron falls from n = 4 to n = 2 is shorter than the wavelength of light emitted when the electron falls from n = 4 to n = 1.
False:
The wavelength of light absorbed when the electron is excited from the ground state to n = 4 is the same as the wavelength of light emitted when the electron falls from n = 4 to the ground state.
The excited state is not the first possible excited state. The first possible excited state is n = 2.
It takes more energy to excite the electron from the n = 4 state than from the ground state because the electron is already further away from the nucleus in the n = 4 state. The electron needs to gain more energy in order to move closer to the nucleus.
On average, the electron is closer to the nucleus in the n = 1 state than in the n = 4 state because the n = 1 state is the lowest energy state. The electron will always be closer to the nucleus in the state with the lowest energy.
The wavelength of light emitted when the electron falls from n = 4 to n = 2 is shorter than the wavelength of light emitted when the electron falls from n = 4 to n = 1. This is because the energy difference between n = 4 and n = 2 is smaller than the energy difference between n = 4 and n = 1. Shorter wavelengths correspond to smaller energy differences.
The wavelength of light absorbed when the electron is excited from the ground state to n = 4 is not the same as the wavelength of light emitted when the electron falls from n = 4 to the ground state. This is because the energy of the absorbed light must exactly match the energy difference between the two states. If the energy of the absorbed light is not exactly right, then the electron will not be excited.
The excited state is not the first possible excited state. The first possible excited state is n = 2. The electron can only move to states with higher energy levels. The n = 1 state is the ground state, which is the lowest energy state.
To know more about wavelength:
https://brainly.com/question/31143857
#SPJ12
A gas has a pressure of 0.75 liters at a pressure of 2.3 X 10 ^4atm. The gas
eventually comes to a pressure of 24.0 atm. What is the volume of the gas after
the explosion?
Answers
Using the formula P₁V₁ = P₂V₂, where P₁ and V₁ are the initial pressure and volume, and P₂ and V₂ are the final pressure and volume, we can solve for V₂.
Given:
P₁ = 2.3 x 10^4 atm
V₁ = 0.75 liters
P₂ = 24.0 atm
Using the formula:
P₁V₁ = P₂V₂
Substituting the values:
(2.3 x 10^4) * 0.75 = (24.0) * V₂
Now we can solve for V₂:
V₂ = (2.3 x 10^4 * 0.75) / 24.0
Performing the calculations, the volume of the gas after the explosion is the result of the above expression.
Elements in the same column of the periodic table of the elements all
Answers
Answer:
Each column is called a group. The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements.
Explanation:
Answer:
the elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons.
~+lil more info +~
The vertical columns (groups) of the periodic table are arranged such that all its elements have the same number of valence electrons. All elements within a certain group thus share similar properties.
b) 25.8 g C12H24012 is burned in an experiment, how many grams of carbon
dioxide is produced?
Answers
Following C12H24012's complete combustion, the following occurs:
C12H24012 + 19O2 → 12CO2 + 24H2O
The amount of carbon dioxide produced is 12 grams.
What is the mass conservation law?
The Law of Conservation of Mass, which states that mass neither creates nor destroys in a chemical reaction, is used in this question.
Steps in this instance are listed below.
1. Calculate C12H24012's molecular weight:
In order to determine the number of moles, divide the given mass of 25.8 g by the molar mass of C12H24012, which is 264 g/mol:
264 g/mol/25.8 g C12H24012 = 0.097 mol
2. Calculate the required oxygen moles:
There are 19 moles of oxygen needed for every mole of C12H24012. In order to determine the necessary amount of oxygen, multiply 0.097 mol by 19:
19 mol O2/1 mol C12H24012 x 0.097 mol C12H24012 = 1.833 mol O2
To learn more about Law of Conservation of Mass refer :
https://brainly.com/question/27891057
#SPJ1
Provide the systematic name for each of the following isomeric acid chlorides with the chemical formula C6H9ClO.
(Be sure to indicate double bond stereochemistry using (E) and (Z) notation. Indicate stereochemistry in rings with the terms cis or trans. Do NOT use (R) or (S) designations. It is not necessary to use italics in writing compound names. Write compound names in lower case. Use upper case for the double bond stereochemistry terms.)
Answers
The systematic names for the isomeric acid chlorides with the chemical formula C6H9ClO are as follows:
cis-3-chlorocyclopent-1-ene-1-carbonyl chloride
trans-3-chlorocyclopent-1-ene-1-carbonyl chloride
2-chloro-3-methylbut-2-ene-1-carbonyl chloride
The first compound is a cis isomer with a chlorine atom and a carbonyl group on the same side of the cyclopentene ring. The prefix "cis-" is used to indicate this stereochemistry.
The second compound is a trans isomer with a chlorine atom and a carbonyl group on opposite sides of the cyclopentene ring. The prefix "trans-" is used to indicate this stereochemistry.
The third compound is a 2-chloro-3-methylbut-2-ene-1-carbonyl chloride. The chlorine atom is attached to the second carbon atom of the butene chain, and the carbonyl group is attached to the first carbon atom.
The prefix "2-chloro" indicates the position of the chlorine atom, and "3-methyl" indicates the presence of a methyl group on the third carbon atom. The term "but-2-ene-1-carbonyl" describes the butene chain with a carbonyl group attached to the first carbon atom.
Overall, these names provide a clear and systematic description of the isomeric acid chlorides based on their molecular structures and stereochemistry.
For more questions like Isomeric acid click the link below:
https://brainly.com/question/29579764
#SPJ4
what drink contains the following ingredients: 2 dashes bitters; 3/4 oz. orange juice; 3/4 oz. dry vermouth; and 3/4 oz. gin?
Answers
The drink that contains 2 dashes of bitters, 3/4 oz. of orange juice, 3/4 oz. of dry vermouth, and 3/4 oz. of gin is called a Satan's Whiskers cocktail. It is a classic cocktail known for its balanced flavors and is enjoyed by cocktail enthusiasts around the world.
The drink that contains the given ingredients of 2 dashes of bitters, 3/4 oz. of orange juice, 3/4 oz. of dry vermouth, and 3/4 oz. of gin is known as a "Satan's Whiskers" cocktail. The Satan's Whiskers is a classic cocktail that comes in two variations: straight and curled. The recipe you provided corresponds to the "straight" variation.
To make a Satan's Whiskers cocktail, you will need the following ingredients:
- 2 dashes of bitters (such as Angostura or orange bitters)
- 3/4 oz. of orange juice
- 3/4 oz. of dry vermouth
- 3/4 oz. of gin
To prepare the cocktail, follow these steps:
1. Fill a cocktail shaker with ice.
2. Add 2 dashes of bitters to the shaker.
3. Pour in 3/4 oz. of orange juice.
4. Add 3/4 oz. of dry vermouth.
5. Finally, pour in 3/4 oz. of gin.
6. Shake the ingredients vigorously for about 15 seconds to combine and chill the drink.
7. Strain the mixture into a chilled cocktail glass.
The Satan's Whiskers cocktail is known for its complex and balanced flavors. The bitters add depth and complexity, while the orange juice provides a refreshing citrusy note. The dry vermouth contributes herbal and slightly bitter flavors, and the gin brings a distinct botanical character to the drink. The combination of these ingredients creates a unique and enjoyable cocktail experience.
For more such information on: cocktail
https://brainly.com/question/29694989
#SPJ8
PLS HELP ME IM GIVING EXTRA POINS I NEED THIS ASAP IM TIMED PLEASE HELP A BROTHER OUT <3
Part 1. Determine the molar mass of a 0. 314-gram sample of gas having a volume of 1. 6 L at 287 K and 0. 92 atm. Show your work.
Part 2. Describe the temperature and pressure conditions at which the gas behaves like an ideal gas
Answers
The molar mass of a 0.314-gram sample of gas having a volume of 1.6L at 287K and 0.92 atm is 0.503g/mol.
The temperature and pressure of a gas that behaves like an ideal gas is directly proportional to one another.
First, calculating molar mass :
The molar mass of a substance can be calculated by dividing the mass of the substance by its number of moles.
However, the number of moles can be calculated using the formula:
PV = nRT
From where;
P = pressure
V = volume
R = gas law constant
T = temperature
n = number of moles
0.92 × 1.6 = n × 0.0821 × 287
1.47 = 23.56n
n = 1.47/23.56
n = 0.624 moles
Molar mass = 0.314 g ÷ 0.624 mol
Molar mass = 0.503 g/mol
Therefore, the molar mass of a 0.314-gram sample of gas having a volume of 1.6L at 287K and 0.92 atm is 0.503g/mol.
Learn more about molar mass here :
brainly.com/question/12127540
#SPJ4
What is the volume of a box that has a length of 5 cm a width of 7 cm and a height of 9 cm
Answers
Answer:
V = L × w × h
V = 5 × 7 × 9
V = 315 cm³
Which conclusion does the passage best support?
The princess is strongly barbaric, so she will choose
the tiger.
The princess loves the young man enough to choose
the lady.
The king is semi-barbaric, so he will make sure the
young man gets the tiger.
The king loves his daughter strongly enough that he
will pardon the young man.
Answers
Answer:
a is the answer
Explanation:
The princess loves the young man enough to choose the lady.
The princess is strongly barbaric, so she will choose the tiger this conclusion does the passage best support.
Why is the princess described as semi-barbaric in The Lady, or the Tiger?The Princess, who, like her father, is semi-barbaric, knows which door hides each creature and directs the young man to the door on the right.
He has grandiose ideas and fancies; he commands that even his most whimsical and unrealistic wishes be fulfilled, and he is burningly, gustily passionate, just like his princess.
Stockton describes the princess, the king's daughter, as intense, jealous, and powerful. In the story, the princess falls in love with a young man. He is of noble blood, but he is beneath her station, so the king summons him to the arena.
Thus, option A is correct.
To learn more about semi-barbaric in The Lady, follow the link;
https://brainly.com/question/18066132
#SPJ6
Formamide decomposes at high temperature. If 0.186 mol of formamide (HCONH2) dissociates in a 2.16 L flask at 400 K, what are the concentrations of all species present at equilibrium at 400 K? (hint: calculate concentrations first) (b) What is the total pressure in the container at equilibrium?
Answers
Answer:
a) [COHNH₂] = 0.001 mol/L, [NH₃] = [CO] = 0.085 mol/L
b) 5.59 atm
Explanation:
a) The decomposition reaction of formamide is the following:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
The equilibrium constant of the reaction above is:
\(K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = 4.84 (400 K)\)
The initial concentration of formamide is:
\( C_{COHNH_{2}} = \frac{\eta}{V} = \frac{0.186 moles}{2.16 L} = 0.086 mol/L \)
Where: η is the number of moles and V is the volume
Now, in the equilibrium the concentration of all species is:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
0.086 - x x x
\( K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = \frac{x*x}{0.086 - x} \)
\( 4.84*(0.086 - x) -x^{2} = 0 \)
By solving the above equation for x we have:
x = 0.085 mol/L = [NH₃] = [CO]
[COHNH₂] = 0.086 - 0.085 = 0.001 mol/L
Therefore, the concentrations of all species present at equilibrium at 400 K is [NH₃] = [CO] = 0.085 mol/L and [COHNH₂] = 0.001 mol/L.
b) To find the total pressure in the container we need to find first the constant Kp as follows:
\( K_{p} = K_{c}*RT^{\Delta n} \)
Where R is the gas constant = 0.082 Latm/(Kmol), T is the temperature = 400 K and Δn = 1
\( K_{p} = K_{c}*RT^{\Delta n} = 4.84*(0.082*400)^{1} = 158.8 \)
Now, the total pressure is:
\( p_{T} = p_{COHNH_{2}} + p_{NH_{3}} + p_{CO} \)
The pressure of COHNH₂ can be found using Ideal Gas Law:
\( P = \frac{nRT}{V} = \frac{0.186 moles*0.082 L*atm/(K*mol)*400 K}{2.16 L} = 2.82 atm \)
Using the equilibrium constant we can find the pressure of NH₃ and CO:
COHNH₂(d) ⇆ NH₃(g) + CO(g)
2.82 - x x x
\( K_{p} = \frac{P_{NH_{3}}*P_{CO}}{P_{COHNH_{2}}} \)
\( 158.8*(2.82 - x) - x^{2} = 0 \)
By solving the above equation for x we have:
\( x = P_{NH_{3}} = P_{CO} = 2.77 atm \)
\( P_{COHNH_{2}} = 2.82 - 2.77 = 0.05 atm \)
Thus, the total pressure is:
\( p_{T} = p_{COHNH_{2}} + p_{NH_{3}} + p_{CO} = (0.05 + 2.77 + 2.77) atm = 5.59 atm \)
Hence, the total pressure in the container at equilibrium is 5.59 atm.
I hope it helps you!
Which best describes a neutralization
reaction?
A) a reaction between an acid and a base
B) a reaction between two acids
C) a reaction between a base and a salt
D) a reaction between two salts
Answers
Explanation:
The Answer is a reaction between two acids
5.0g of copper is heated from 20 Celsius to 80 Celsius. How much energy it was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g)
Answers
Answer:
the energy used to heat Cu is 27.6 Cal
Explanation:
given data
mass m = 5 g
Specific heat capacity of Cu = 0.092 cal/g
temperature T2 = 80 Celsius
temperature T1 = 20 Celsius
solution
we get here energy used to heat Cu that is express as
Q = m × c × ΔT ....................1
so here m = mass and c = specific heat capacity
and ΔT = T2 - T1
so put value in eq 1
Q = 5 × 0.092 × (80 - 20)
Q = 27.6 Cal
so that the energy used to heat Cu is 27.6 Cal
Which is the right answer
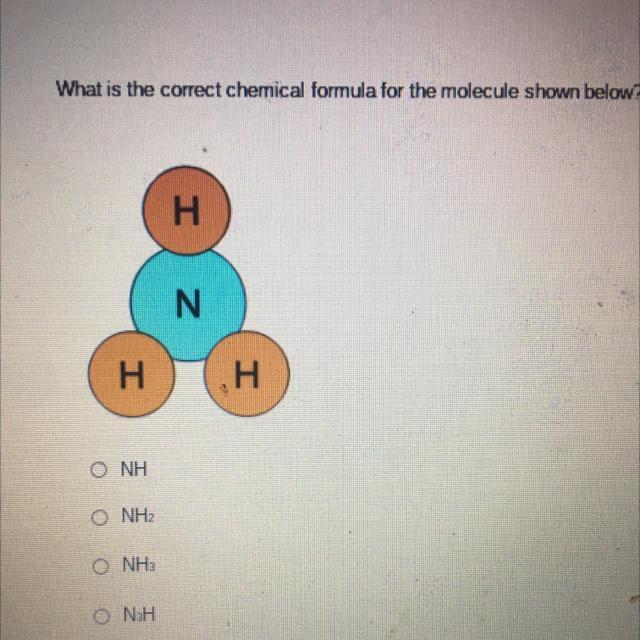
Answers
Answer:
NH3
Explanation:
there is one nitrogen and 3 hydrogen
calculate amount of tin and mass percentage

Answers
Amount of tin in the sample is 2.2837 gm and its mass percentage in the rock is 22.837%
I₃⁻ (aq) + Sn⁺² (aq) → Sn⁺⁴ (aq) + 3I⁻
1 mol 1 mol
mol of NaI₃ = 34.60 mL × 1 L / 1000 mL × 0.5560 mol / L
= 19.2376 × 10⁻³ mol
∵ 1 mol of Sn²⁺ = 1 mol of I₃⁻
∴ mol of Sn²⁺ = 19.2376 × 10⁻³
∴ mass of Sn in sample = mol of Sn²⁺ × molar mass
= ( 19.2376 × 10⁻³ mol ) × 118.71 gm/mol
= 2.28369 gm
= 2.2837 gm
∴ % of tin in sample = (mass of tin) / (mass of sample ) × 100
The mass percent of a solution is defined as the ratio of the mass of solute that is present in a solution, relative to the mass of the solution
= 2.2837/10 × 100
= 22.837 %
Thus we can conclude that amount of tin in the sample is 2.2837 gm and its mass percentage in the rock is 22.837%
Learn more about mass percentage at https://brainly.com/question/5493941
#SPJ1
What is the maxlmum number of electrons that can be present in each principal energy level of hydrogen?
Answers
Answer:
Two electrons
Explanation:
There are 75.0 g of glucose (molar mass = 180 g/mol) dissolved in 250 mL of solution. What is the molarity of this solution?
A. 0.416 M
B. 1.70 M
C. 2.13 M
D. 3.31 M
Answers
Answer:
5.6 m which is molarith this solution
a student collected data about how different fertilizers affect a plant growth. which type of graph should they use to show the data? PLS HELP ASAP :C worth 100 points lol
Answers
Answer:
I think they should use a bar graph
Explanation:
Because if they use a bar graph they could mark each of the different kind of fertilizer and they could easily see which one affected it the most, either good or bad, by the looking at the heights of the bars to determine your conclusion.
Hi there!
:bar graph:
If they use a bar graph they could mark each of the different kind of fertilizer and they could see easier to tell which one affected it the most, by looking at the heights of the bars to determine the answers.
Calculate the activity of 1 gram U3Si2 [in µCi], where uranium is 5% (by mass) enriched in 235U and silicon is stable as 28Si. Ans. 0.394 MCi
Answers
The activity of 1 gram of U3Si2, where uranium is 5% enriched in 235U, is calculated to be 0.394 MCi (megaCuries).
To calculate the activity, we need to consider the radioactive decay of the 235U isotope in U3Si2. The activity can be determined using the decay constant (λ) and the number of atoms of the radioactive isotope present.
Since uranium is 5% enriched in 235U, we can calculate the number of atoms of 235U in 1 gram of U3Si2. Then, using the decay constant for 235U, we can determine the activity.
The detailed calculations involve determining the number of moles of 235U in 1 gram of U3Si2, converting it to the number of atoms, and then multiplying by the decay constant and Avogadro's number to obtain the activity in curies. The final result is 0.394 MCi, indicating the rate of radioactive decay for the given sample.
To know more about Avogadro's number click here: brainly.com/question/28812626
#SPJ11
a doctor examines a mole with a 15.0 cm focal length magnifying glass held 10.4 cm from the mole. what is its magnification?
Answers
The magnification of the magnifying glass is approximately 1.44.
To calculate the magnification of the magnifying glass, we can use the formula:
Magnification = (image distance) / (object distance)
In this case, the magnifying glass is held 10.4 cm from the mole, so the object distance (d₀) is 10.4 cm. The focal length (f) of the magnifying glass is given as 15.0 cm.
Using the formula, we can calculate the magnification (M):
M = (image distance) / (10.4 cm)
The magnification of a magnifying glass is typically assumed to be the ratio of the image distance to the least distance of distinct vision (25 cm for a relaxed eye). Therefore, we need to find the image distance (dᵢ) in terms of the least distance of distinct vision.
Using the lens formula: 1/f = 1/d₀ + 1/dᵢ
We can rearrange the formula to solve for the image distance (dᵢ):
1/dᵢ = 1/f - 1/d₀
Substituting the given values:
1/dᵢ = 1/15 cm - 1/10.4 cm
Calculating the result:
1/dᵢ ≈ 0.0667 cm⁻¹
dᵢ ≈ 15 cm
Now we can substitute the values for image distance (dᵢ) and object distance (d₀) into the magnification formula:
M = 15 cm / 10.4 cm
Simplifying the calculation:
M ≈ 1.44
Therefore, the magnification of the magnifying glass in this scenario is approximately 1.44.
To learn more about magnification, click here:
https://brainly.com/question/1599771
#SPJ11
Biodiversity is important for the sustainability of ecosystems. However, many of the human actions that are aimed at growing communities are decreasing the biodiversity of these areas, leaving populations and ecosystems vulnerable. As human populations grow, the terrestrial and aquatic ecosystems they use may be transformed by the efforts of human beings and biodiversity losses typically accompany these processes. Which of these actions decrease biodiversity and threaten sustainability? Select ALL that apply.
A Invasive (non-native) species may outcompete native species for food and habitat. Invasive (non-native) species may outcompete native species for food and habitat.
B Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions. Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.
C Pollution from building and commercialization can create health problems in exposed organisms. Pollution from building and commercialization can create health problems in exposed organisms.
D Decreased use of pesticides and fertilizers can allow for increased damage to plant life. Decreased use of pesticides and fertilizers can allow for increased damage to plant life.
E Habitat destruction reduces or eliminates the food resources and living space for most species. Habitat destruction reduces or eliminates the food resources and living space for most species.
Answers
The actions by humans that decrease biodiversity and threaten sustainability are;
Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.Pollution from building and commercialization can create health problems in exposed organisms.Habitat destruction reduces or eliminates the food resources and living space for most species.What is biodiversity?The term biodiversity has to do with the existence of different species that exist in an ecosystem. In a given ecosystem, there could be several species that exist together. This biodiversity is very much important for the continuity of balance in nature.
The actions by humans that decrease biodiversity and threaten sustainability are;
Regulated hunting seasons artificially decrease hunted populations, often leading to extinctions.Pollution from building and commercialization can create health problems in exposed organisms.Habitat destruction reduces or eliminates the food resources and living space for most species.Learn more about biodiversity:https://brainly.com/question/13073382
#SPJ1
Calculate the mass of water present in a 5.75 molal solution made with 135.0-grams of thiourea (CHAN2S).
Answers
The mass of water present in the solution is approximately 13.996 grams.
To calculate the mass of water present in a 5.75 molal solution made with 135.0 grams of thiourea (CH4N2S), we need to first determine the moles of thiourea and then use the molality to find the moles of water.
The molar mass of thiourea (CH4N2S) can be calculated as follows:
(1 * 12.01 g/mol) + (4 * 1.01 g/mol) + (2 * 14.01 g/mol) + (1 * 32.07 g/mol) = 76.12 g/mol
Next, we can calculate the moles of thiourea:
Moles of thiourea = mass of thiourea / molar mass of thiourea
Moles of thiourea = 135.0 g / 76.12 g/mol = 1.774 mol
Since the molality of the solution is 5.75 molal, it means that there are 5.75 moles of solute (thiourea) per kilogram of solvent (water).
Now, we can calculate the moles of water:
Moles of water = molality * mass of solvent (in kg)
Moles of water = 5.75 mol/kg * (135.0 g / 1000 g/kg) = 0.7774 mol
Finally, we can determine the mass of water:
Mass of water = moles of water * molar mass of water
Mass of water = 0.7774 mol * 18.015 g/mol = 13.996 g
For more such questions on water visit:
https://brainly.com/question/30173238
#SPJ8