Answers
Answer:
All of the above are correct.
Explanation:
The Flame Test Shortcomings
Small quantities of most molecules can not be measured by the examination.
The signal's intensity ranges from one test to the next.
The test findings are impaired by impurities or toxins.
The test is not capable of differentiating between all components.
Related Questions
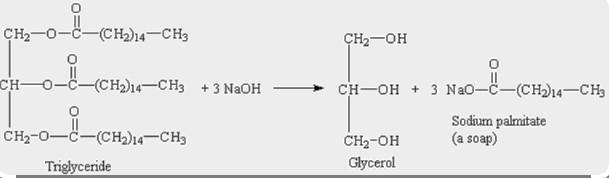
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
aromatic compounds aliphatic compounds
Answers
Answer:
I hoped it helps you fod blessed:)

A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures wide and high. The maximum safe pressure inside the vessel has been measured to be .
For a certain reaction the vessel may contain up to of carbon monoxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits.
Answers
Answer:
T = 643K = 643-273.15 = 369.85°C = 370°C = maximum safe operating temperature
Explanation:
The actual question has following data which the question given has missing
The vessel is a stainless-steel cylinder that measures wide 25.0 cm and 30.0 cm high.
The maximum safe pressure inside the vessel is 6.60 MPa.
The vessel may contain up to 0.800 kg of carbon monoxide gas.
Volume of cylinder = pi x r^2 x h = 3.14 x (25/2)cm^2 x 30cm = 14725.78 cm^3 or 0.01472578 m^3
Now, using ideal gas law, PV = nRT
P = 6.60×10^6 Pa
V = 0.01472578 m^3
n= no. of moles = mas staken/ molar mass of CO2 = 800g/44g/mol = 18.18
moles
R = 8.314 J/mol.K
So, (6.60x10^6 ) x 0.01472578 = 18.18×8.314×T
T = 643 K = 643-273.15 = 369.85°C = 370°C = maximum safe operating temperature
Write a balanced equation for the reaction which occurs with the CaCI2 solution and the soap (a fatty acid salt). **Use “(fatty acid-CO2)-Na+” as the structure for the soap instead of drawing out the entire fatty acid structure
Answers
HCl balancing the formula is as follows: CaC\(O_{3}\) + HCl Cd(ii 2 + Hydrogen ion O \(CO_{2}\). By substituting 2 moles of HCl on the LHS with 1 mole of \(H_{2}0\) on the RHS, the equation is made equal.
What is hydrochloric acid, sometimes known as HCl?
Hydrochloric acid (HCl Acid): Structure, Atomic Mass, Preparations, Properties, and Videos with Acid hydrochloride is an inorganic substance. With the organic compound with the formula HCl, it is a very corrosive acid. Muriatic acid and hydrogen chloride are other names for it.
How is \(H_{2}\) \(Cl_{2}\) HCl balanced?
how to balance Hydrogen + \(Cl_{2}\) HCl. Make sure to count every H and Cl atom upon every side of the molecular formula in order to attain equilibrium \(H_{2}\) + \(Cl_{2}\) HCl. You can only alter the coefficient (the numbers between the atoms or combinations) to balance the formula once you are aware of the quantity of each sort of atom you have.
To know more about HCl balancing visit:
brainly.com/question/29329799
#SPJ1
A 108.9 g sample of water absorbs 114.6 calories of heat. The specific heat capacity of water is 1 cal/(g·°C). By how much did the temperature of this sample change, in degrees Celsius?
Answers
Answer:
The temperature of this sample changes by 1.052 degrees Celsius
Explanation:
As we know
\(Q = mc\Delta T\)
Where m is the mass of the substance
c is the specific heat of the substance
and \(\Delta T\) is the change in temperature
Substituting the given values in above equation, we get -
\(114.6 = 108.9 * 1 * \Delta T\\\Delta T = 1.052\)degree Celsius
The temperature of this sample changes by 1.052 degrees Celsius
12.
Which of the following does not indicate that a chemical reaction occurred when the iron filings
were added to the copper(II) sulfate?
Answers
You didn't provide the answer choices so I'll give all that I know.
No color change occurs
No odor is produced
No gas is produced
No temperature change occurs(Stays at room temp)
No precipitation occurs
PLEASE HELP ME WITH THE QUESTION IN THE IMAGE BELOW I WILL MAKE YOU BRAINLIEST AND GIVE YOU 17 POINTS!

Answers
Answer:
The sound playing from the first graph is louder, while the sound from the second graph is deeper, both of which have the same pitch.
Explanation:
First let's analyze the graphs,
Comparing the first graph to the second
Concluding from the above observations, the sound playing from the first graph is louder, while the sound from the second graph is deeper, both of which have the same pitch.
What is meant by H, and 2H?
Answers
Explanation:
H is 1 mole of hydrogen
2H is 2 moles of hydrogen
A constant electric current deposited 365 mg of Ag in 216 minutes from an aqueous Silver trioxonitrate (v). What is the Current?
Answers
The electric current is 0.025 A
Electric current refers back to the go with the flow of energy in an electronic circuit and to the amount of strength flowing through a circuit. it's far measured in amperes (A). the bigger the cost in amperes, the more energy is flowing within the circuit.
Ag+ + e¯ →Ag
1F deposits 107.87 g/mol (molecular mass) of silver
1F = 96500 C
Let, 107.87 g/mol needed = 96500 C
Number of coulombs required to deposit 0.3650 g of silver =(96500/107.87) 0.3650
Q = 326.5 C
According to Faraday’s law, Q = I x t
I = 326.5 C / (216 x 60 s) = 0.025 A
Learn more about electric current here:-https://brainly.com/question/2984202
#SPJ9
A sample of an ideal gas has a volume of 3.70 L at 12.20 ∘C and 1.40 atm. What is the volume of the gas at 20.80 ∘C and 0.989 atm?
Answers
The volume of the given gas is 5.35 L.
Here we use the combined gas law viz. P1V1/T1 = P2V2/T2, which is derived from the ideal gas equation PV = nRT, where
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature.
Here,
\($P_1\) = 1.40 atm, \($P_ 2\)= 0.989 atm
\($V_1\) = 3.70 L, \($V_2\) = ?
\($T_1\) = 12.20°C = (12.20 + 273) K = 285.2 K
\($T_2\) = 20.80°C = (20.80 + 273) K = 293.8 K
substituting the values in the equation P1V1/T1 = P2V2/T2, we get,
(1.40 x 3.70)/285.2 = 0.989 x \($V_2\)/293.8
\($V_2\) = (1.40 x 3.70 x 293.8) / (285.2 x 0.989)
\($V_2\) = 1521.884 / 284.211
\($V_2\) =5.35 L
Thus, the volume of the gas is 5.35 L.
Read more about the Combined gas law:
brainly.com/question/25587265
I need to find the average percent recovery

Answers
Consider that after recrystallization, you obtained 7.0 g of dry pure substance from 10.0 g of impure material. Your recovery rate is then 70% (7/10 × 100).
What is the typical recrystallization percentage?Recrystallization recovery percentages are often lower than 100%, though occasionally they might be higher (see the following issue). This is brought on by impurity loss, material that was left in the liquid solution after dissolving, and "mechanical losses."
What drives our % recovery calculations?The amount if pure compound that is present in the finished chemical synthesis product is calculated using the percent recovery. The ratio of actual yield to theoretical yield is used to compute percent yield. The ratio of the pure compound to the starting compound is used to calculate percent recovery.
To know more about material visit:
https://brainly.com/question/28395976
#SPJ1
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
A particular term in an atom in which LS coupling is a good approximation splits into three levels, each having the same L and same S but different J. If the relative spacings between the levels are in the proportion 5:3, find L and S.
Answers
Answer:
Explanation:
From the information given;
Consider using Lande's Interval rule which can be expressed as:
\(\Delta E = E_{j+1} - E_jj \ = \alpha (j+1)\)
here;
\(j+1\) = highest level of j
and
\(\dfrac{\Delta E_1}{\Delta E_2} = \dfrac{(j+2)}{(j+1)}\)
\(\dfrac{5}{3} = \dfrac{(j+2)}{(j+1)}\)
\(5(j+1) = 3(j+2)\)
\(5j+5 = 3j+6\)
\(2j = 1\\ \\ j = \dfrac{1}{2}\)
recall that:
\(j = |S-L| \ \to \ |S+L |\)
So;
\(S-L = \dfrac{1}{2} --- (1)\); &
\(S+L = \dfrac{5}{2} --- (1)\)
Using the elimination method, we have:
\(2S = \dfrac{6}{2}\)
\(S = \dfrac{3}{2}\)
Since \(S = \dfrac{3}{2}\); then from (1)
\(\dfrac{3}{2} -L = \dfrac{1}{2}\)
\(L = \dfrac{2}{2}\)
\(L = 1\)
Silver sulfate is precipitated from solution according to the following reaction 2 AgNO3 (aq) Na2SO4 (aq) --> Ag2SO4 (s) 2 NaNO3 (aq) If 3.80 moles of AgNO3 and 3.05 moles of Na2SO4 are present initially, how many moles of excess reactant will remain after precipitation is complete
Answers
Answer:
1.15 moles of excess reactant will remain after precipitation is complete.
Explanation:
The balanced reaction is:
2 AgNO₃ (aq) + Na₂SO₄ (aq) → Ag₂SO₄ (s) + 2 NaNO₃ (aq)
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
AgNO₃: 2 molesNa₂SO₄: 1 moleAg₂SO₄: 1 moleNaNO₃: 2 molesThen you can apply the following rule of three:: if by stoichiometry 2 moles of AgNO₃ reacts with 1 mole of Na₂SO₄, 3.80 moles of AgNO₃ reacts with how much moles of Na₂SO₄?
\(amount of moles of Na_{2}SO_{4} =\frac{1mole of Na_{2}SO_{4} * 3.80 moles of AgNO_{3} }{2 mols of AgNO_{3} }\)
amount of moles of Na₂SO₄= 1.9 moles
But 3.05 moles of Na₂SO₄ are available. Since you have more moles than you need to react with 3.80 moles of AgNO₃, Na₂SO₄ will be the excess reagent.
To calculate the amount of excess reagent that will remain, you must make the difference between the amount you initially have and the amount that reacts:
3.05 moles - 1.9 moles= 1.15 moles
1.15 moles of excess reactant will remain after precipitation is complete.
Which of these are polar SF2 SF4 and SF6
Answers
Answer: SF2 & SF4
Explanation:
Molecular Geometry
SF2 : bend
SF4 : seesaw
SF6 : octahedral
What is the balanced chemical equation for the reaction used to calculate ΔH∘f of BaCO3(s) ?
Answers
The balanced equation that can be used to fond the heat of formation is;
\(Ba(s) + C(s) + \frac{3}{2}O_{2} (g) ----- > BaCO_{3} (s)\)
What is the heat of formation?The heat of formation is defined as the heat that is evolved or absorbed when a substance is formed from its component elements under standard conditions. Note that the components of the equations must be the substances that are in standard form.
In this case, we are trying to see the balanced chemical equation for the reaction used to calculate ΔH∘f of BaCO3(s). We have to know that is equation must have to involved the barium, carbon and oxygen atoms as the barium carbonate compound is formed.
Learn more about heat of formation:https://brainly.com/question/13096727
#SPJ1
4. Hydrogen bonds, ionic bonds, dipole-dipole attractions, dispersion forces are types intermolecular forces.Indicate the major type of intermolecular forces that occur between particles in the following substances: 6 pts(a) OBr2 =(b) LICI(c) H20 -(d) HBr -(e) CHCI) =( 1Br =

Answers
Answer:
a) Dipole-Dipole
b) Ionic Bond
c) Hydrogen Bond
d) Dipole-Dipole
e) Dipole- Dipole
f) Dispersion Forces
Explanation:
Here, we want to get the type of intermolecular forces that exist in the given molecules
Hydrogen bond exists when we have hydrogen being bonded to highly electronegative atoms.
Ionic Bonds exist between electropositive and electronegative atoms in a molecule
Dipole-Dipole interaction is a weak type of intermolecular force arising from induced diploes
Dispersion forces forms from temporary dipoles and they are weak
The molecules and their type of intermolecular forces are as follows:
\(\begin{gathered} a\text{ \rparen OBr}_2\text{ - Dipole - Dipole Interaction} \\ b)\text{ LiCl - Ionic Bond} \\ c)\text{ H}_2O\text{ - Hydrogen Bond} \\ d)\text{ HBr - Dipole -Dipole Interaction} \\ c)\text{ CHCl}_3\text{ - Dipole-Dipole Interaction} \\ f)\text{ IBr - Dispersion Forces} \end{gathered}\)We should note that Dipole-Dipole forces occur between atoms that are electronegative
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
the table below gives the atomic number of elements w x and y and z.The the letters do not represent the actual symbols of the elements .
W. X Y. Z
9. 10. 11. 12
which one of the element is less reactive explain .
Answers
Element w is less reactive than elements x, y, and z. The element with the lower atomic number is typically less reactive.
Element w has an atomic number of 9, element x has an atomic number of 10, element y has an atomic number of 11, and element z has an atomic number of 12. Based on this information, we can conclude that element w is less reactive than elements x, y, and z.
This is because the reactivity of an element is largely determined by the number of valence electrons it has. Valence electrons are the electrons in the outermost shell of an atom that are involved in chemical reactions. Elements with fewer valence electrons are less reactive because they are more stable. Element w has only one valence electron, while elements x, y, and z have two, three, and four valence electrons, respectively.
In general, elements with a full outermost shell of electrons, such as the noble gases, are the least reactive because they are highly stable. Elements that are close to having a full outermost shell, such as element w, are also relatively stable and less reactive. On the other hand, elements with only a few valence electrons, such as the alkali metals, are highly reactive because they are trying to gain or lose electrons in order to achieve a full outermost shell.
Overall, the reactivity of an element is determined by its electronic structure, with elements having fewer valence electrons generally being less reactive than those with more. In the case of the elements w, x, y, and z, we can see that element w has the fewest valence electrons and is therefore the least reactive.
For more such questions on Element
https://brainly.com/question/28376204
#SPJ11
The specific heat of copper metal was determined by putting a piece of the metal weighing g in hot water. The quantity of heat absorbed by the metal was calculated to be J from the temperature drop of the water. What was the specific heat of the metal if the temperature of the metal rose
Answers
Answer:
J/g°C=H
H×g°C=J
H×g°C/H×g=J/H×g
A brick has a mass of and the Earth has a mass of . Use this information to answer the questions below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of bricks? Round your answer to 2 significant digits. How many moles of bricks have a mass equal to the mass of the Earth? Round your answer to 2 significant digits.
Answers
The given question is incomplete. The complete question is:
A brick has a mass of 4.0 kg and the earth has a mass of \(6.0\times 10^{27}g\) Use this information to answer the questions below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of bricks? Round your answer to 2 significant digits. How many moles of bricks have a mass equal to the mass of the Earth? Round your answer to 2 significant digits.
Answer: The mass of 1 mole of bricks is \(2.4\times 10^{27}g\)
2.5 moles of bricks have a mass equal to the mass of the Earth
Explanation:
According to avogadro's law, 1 mole of every substance weighs equal to its molecular mass and contains avogadro's number \(6.023\times 10^{23}\) of particles.
Mass of 1 brick = 4.0 kg = 4000 g (1kg=1000g)
Thus mass of 1 mole of brick or \(6.023\times 10^{23}\) bricks will be = \(\frac{4000g}{1}\times 6.023\times 10^{23}=2.4\times 10^{27}g\)
The mass of 1 mole of bricks is \(2.4\times 10^{27}g\)
Now \(2.4\times 10^{27}g\) will correspond to = 1 mole of bricks
\(6.0\times 10^{27}g\) will correspond to =\(\frac{1}{2.4\times 10^{27}}\times 6.0\times 10^{27}=2.5moles\)
2.5 moles of bricks have a mass equal to the mass of the Earth
Which of the following is a true statement?
A)Temperature is a constant condition with respect to Charles's Law
B)Charles's Law involves the units of volume and pressure
The relationship between the units involved in Boyle's Law is one of exponential decay
C)The relationship between the units involved in Charles Law is exponential growth
D)None of these statements are true
Answers
Answer:
B) Charles's law involves the units of volume and pressure
The relationship between the units involved in Boyle's law is one of the exponential Decay
Explanation:
follow me for more of my answers
Air will expand about the same amount as propane with the same change in temperature over ordinary temperature ranges.
Answers
Answer:
Yes this is true
Explanation:
Answer:
i have the same question for chemistry, do you have the answer?
Explanation:
You give a soccer ball a hard kick on the grass. The ball eventually comes to a stop. Which force stops the soccer ball from moving?
A. Gravity
B. Friction
C. Pull
C. Push
Answers
Answer: Friction
Explanation: It is Friction because force acts in the opposite direction to the motion of the ball slowing it and eventually stopping it.
prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8
2.Which of the following is NOT an example of compound?
a. Ammonia
b.Helium gas
c. Hydrogen peroxide
d. Table sugar
3.Which of the following statements in TRUE about alements and compounds?
a. Are homologous in nature
b. Are simplest from matter
c. Are commonly naturally occurring materials
d. Can be broken down into simpler substances
4.Which of the following substance is an element?
a.carbon dioxide
b.iron
c.salt
d.sugar
5.Which of the following describes an element?
a.the simplest substance
b.It can be broken down into other types of substance
c. it can be separated through a chemical process
d.It is composed of two or more types
6.Which of the following statements correctly describes a compound?
a. can be broken down into a simpler type of matter by chemical means.
b.has a unique property that are different from the properties of it's individual elements
c. composed of atoms of two or more elements that bond together.
d.composed of two atoms that bond together
7.which of the following will be teh result of compound if tydrogen gas and oxygen gas combine?
a.alcohol
b.salt
c. sugar
d.water
8.salt in made up of what elements?
a.sodium of Chloride
b. sodium of chlorine
c.sodium and copper
d.sodium and oxide
9. which of the following is correctly matched?
a.Gold: Element: Silver: Compound
b.Oxygen: Element:Water: Compound
c.Sugar: Element:Salt : Compound
d. Water: Element: Hydrogen: Compound
10.which of the following is NOT an element?
a. na
b. Fe
c. pz
d.he
For items 11-15 whether it is an example of an element or a compound. Write the word ELEMENT if the sample shows characteristics of an element and COMPOUND if it shows characteristics of compound.
11.Aluminum
12. Mercury
13. Forrous Sulfate
14.Sucrose
15.Carbon
Answers
A compound is composed of two or more elements. Elements are composed of atoms.
In question 2, we can see that Helium gas is not a compound. A sample of Helium is made up of individual atoms of helium which are not chemically combined.
In question 3, the true statement about elements and compounds is that they are homologous in nature.
In question 4, the element mentioned among the list is iron. It is not chemically combined.
In question 5, The statement that describes an element is that it is the simplest substance. Elements have separate existence.
In question 6, the statements that correctly describes a compound ar;
has a unique property that are different from the properties of it's individual elementscomposed of two atoms that bond togetherIn question 7, When hydrogen and oxygen gas combine, the result is water.
In question 8, a salt is made up of sodium and chlorine. This is our common table salt.
The correct matches for question 9 are;
Gold: ElementOxygen: Element:Water: CompoundSalt : CompoundIn question 10, the symbol that does not depict an element is pz.
For question 11, the correct matches;
Aluminum: ELEMENT
Mercury: ELEMENT
Ferrous Sulfate: COMPOUND
Sucrose: COMPOUND
Carbon: ELEMENT
Learn more: https://brainly.com/question/13516179
heating curve iron
at what temperature does the substance begins to boil
at what temperature does a substance begin to melt
at what temperature is a substance for a liquid and a gas
at what temperature is the substance both a solid and a liquid

Answers
The substance begins to boil at 2750⁰C, the substance begins to melt at 1500⁰C, the temperature at which the substance is both a liquid and a gas at 2750⁰C, temperature is the substance both a solid and a liquid at 1500⁰C.
Heating curves are the graphical correlations between heat added to a substance. When viewed from a cooling perspective, ie. loss of heat, it is the cooling curve.
The gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. The more heat is required to change the temperature of the substance, the slower it cools, so the smaller the gradient of the curve. The higher the thermal conductivity, the faster heat is transferred, so the faster the substance cools.
Learn more about Heating curve, here:
https://brainly.com/question/29592874
#SPJ1
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
One method for preparing a nitrile is the dehydration of a primary amide.
a. True
b. False
Answers
What will most likely cause a sudden wind to start blowing? Help ;-;
A. Clouds
B. Humidity
C. Precipitation
D. Air pressure
Answers
it is D. Air pressure!!! :)