what is the frequency of light having an energy of 1.93 x 10-17 j (h = 6.63 x 10-34 j s and c = 2.99 x 108 m)?
Answers
The frequency of light having an energy of 1.93 x 10⁻¹⁷ J is 2.91 x 10¹⁶ Hz.
The relationship between the energy of a photon (E), its frequency (ν), and the Planck constant (h) is given by the equation E = hν.
Therefore, we can rearrange this equation to solve for the frequency: ν = E/h.
Substituting the given values, we get ν = (1.93 x 10⁻¹⁷ J)/(6.63 x 10⁻³⁴ J s) = 2.91 x 10¹⁶ s⁻¹.
However, we need to convert this value from s⁻¹ to Hz, which is the unit for frequency.
Since Hz is defined as one cycle per second, we have:
ν(Hz) = ν(s⁻¹) = 2.91 x 10¹⁶ s⁻¹ / 1 s = 2.91 x 10¹⁶ Hz
To know more about frequency, refer here:
https://brainly.com/question/20386238#
#SPJ11
Related Questions
which statement best rationalizes ssys for combustion of methane? ch4(g) 2o2(g) co2(g) 2h2o(g) group of answer choices this is a combustion reaction so ssys will be a large negative number. this is a combustion reaction so ssys will be a large positive number. combustion reactions are exothermic, so ssys also will be a large negative number there are no changes of state and no change in nrxn so ssys will be small.
Answers
The entropy of the system (Ssys) for the combustion of methane will be a large negative number. This is consistent with the third statement, which correctly rationalizes the relationship between combustion reactions and the entropy change.
The third statement "combustion reactions are exothermic, so ssys also will be a large negative number" best rationalizes the entropy of the system (Ssys) for the combustion of methane.
Combustion of methane is an exothermic reaction, which means it releases heat to the surroundings. In exothermic reactions, the products have lower potential energy than the reactants, and the system loses energy. This loss of energy in the form of heat corresponds to a decrease in entropy, as the molecules become more ordered and the number of possible energy states decreases.
For such more questions on Entropy:
https://brainly.com/question/30481619
#SPJ11
The simulation opens on the tab labeled Bar Magnet. It shows a magnetic compass and a bar magnet. Move the compass next to each end of the magnet. How do the poles of the compass needle interact with the bar magnet’s south (S) and north (N) poles?
Answers
The earth is a magnet and thus attracts the metallic needle of the compass b the magnetic needle of the compass aligns itself with the Earth's magnetic field C The Earth's south pole lacks.
Why does bar magnet always point in north direction?Because the earth behaves like a magnet, with its south pole in the geographical north and the north pole in the geographical south, a freely suspended magnet points north-south.
Because the compass needle's north seeking pole is always drawn to the north, the earth must be like a massive magnet with a magnetic pole at each end.
Thus, This is true, but magnetic north differs slightly from the earth's north axis of rotation.
To learn more about the bar magnet, follow the link;
https://brainly.com/question/18403251
#SPJ1
12. If a lab experiment is not completed, you should... A. Discuss the issue with your instructor. B. Sneak in after school and work alone. C. Come in during lunch and finish while eating. D. Make up some results. • 13. You are heating a substance in a test tube. Always point the open end of the tube.... A Towed yourself B. Toward your lab partner. C. Toward another classmate. D. Away from all people. . 14. You are heating a piece of glass and now want to pick it up. You should... A. Use a rag or paper towels. B. Pick up the end that looks cooler. C. Use Tongs. D. Ask someone else to pick it up . 15. You have been injured in the laboratory (cut, burn, etc.). First you should... A. Visit the nurse after class. B. See a doctor after school. C. Tell the Lab Instructor at once. D. Apply first aid yourself. • 16. When using glassware and equipment for an experiment, you should... A. Read all directions carefully to know what equipment is necessary. B. Examine all glassware to check for chips or cracks. C. Clean any glassware that appears dirty. D. All of the above. • 17. If a piece of equipment is not working properly, stop, turn it off, and tell... A. The cleaning helper. B. Your lab partner. C. Your best friend in the class. D. The Lab Instructor. . 18. If an acid is splashed on your skin, wash at once with... A Soap. B. Oil. C. Weak base. D. Plenty of water.
Answers
Laboratory environments can be dangerous because of the existence of explosive compounds, potentially toxic microbes, volatile solvents, high pressure gases, and hazardous and caustic substances.
What is safety in the laboratory?The presence of hazardous and caustic substances, volatile solvents, high pressure gases, explosive compounds, and potentially harmful bacteria in a laboratory raises safety concerns. Mistakes in the lab can be avoided with a little caution and respect to established safety requirements.
Make sure you are protected from any splashes by covering up any exposed skin with clothing and footwear. Keep long hair, jewellery, and other anything that could get caught in gear in a ponytail. Never use cosmetics (including lip balm) or contact lenses in a lab setting, and never eat, drink, chew gum, or consume liquids.
Suitable facilities and equipment, suitable training, PPE, chemical management, standard operating procedures, waste disposal, signage, proper laboratory practices, and secure working conditions are all part of good laboratory safety measures.
Therefore, the answers are:
12. a)discuss the issue with your instructor.
13. D)away from all people.
14. c)use tongs.
15. You were hurt in the laboratory (cut, burn, etc.) Visit the school nurse after class as your first course of action. after school, visit a doctor.
16. a) Read all directions carefully to know what equipment is necessary.
17. d) The Lab instructor.
18. d) Plenty of water.
To learn more about safety in the laboratory refer to:
https://brainly.com/question/17994387
#SPJ4
What is carbon? What does it do?
Answers
How many grams in 1.000 moles of oxygen gas?
Answers
An avocado contains 234 Calories, with 21 grams of fat. Calculate the percent of Calories from fat.
Answers
Please HELP due for science

Answers
Answer: The specimens appears larger with a higher magnifications because a smaller area of the object is spread out to cover the filed view of your eye.
Explanation:
What are the two types of carbohydrates and food sources?.
Answers
Answer: simple and complex.
Explanation:These are also called simple sugars. They're found in refined sugars, like the white sugar you see in a sugar bowl. If you have a lollipop, you're eating simple carbs.
For the reaction Pb(NO3)2 + 2KI = PbI2 + 2KNO3 , how many moles of PbI2 are produced from 600. G KI?
a. 563 g
b. 55. 7 g
c. 181 g
d. 833 g
Answers
The closest option is (c) 181 g, which represents 1 mole of PbI2. The moles of \(PbI_2\) produced from 600 g of KI is approximately 1.807 moles.
To determine the moles of \(PbI_2\) produced from 600 g of KI, we first need to find the molar mass of KI and \(PbI_2\).
Molar mass of KI:
K = 39.10 g/mol
I = 126.90 g/mol
Molar mass of KI = 39.10 g/mol + 126.90 g/mol = 166.00 g/mol
Next, we calculate the number of moles of KI in 600 g:
Moles of KI = mass of KI / molar mass of KI
= 600 g / 166.00 g/mol
= 3.614 moles
From the balanced chemical equation, we can see that the stoichiometric ratio between KI and \(PbI_2\) is 2:1. Therefore, for every 2 moles of KI, we get 1 mole of \(PbI_2\).
Moles of \(PbI_2\) = Moles of KI / 2
= 3.614 moles / 2
= 1.807 moles
Therefore, the moles of \(PbI_2\) produced from 600 g of KI is approximately 1.807 moles.
Based on the answer choices given, the closest option is (c) 181 g, which represents 1 mole of \(PbI_2\).
Learn more about moles :
https://brainly.com/question/26416088
#SPJ11
About how much of a star's life is spent as a main-sequence star?
Answers
Eighty Billion Years
Which of the following statements concerning mixtures is correct?
a. The composition of a homogeneous mixture cannot vary.
b. A homogeneous mixture can have components present in two physical states.
c. A heterogeneous mixture containing only one phase is an impossibility
d. More than one correct response..
Answers
The correct option from the given statements concerning mixtures is (d) more than one correct response.
The statement (a) "The composition of a homogeneous mixture cannot vary" is incorrect as the composition of a homogeneous mixture can vary. For example, a mixture of salt and water is homogeneous and its composition can vary depending on the amount of salt and water mixed in it.
The statement (b) "A homogeneous mixture can have components present in two physical states" is correct. Homogeneous mixtures are mixtures that are uniform throughout their composition, meaning that there is no visible difference between the components of the mixture. For example, a mixture of ethanol and water is homogeneous and its components are present in two physical states (liquid and liquid).
The statement (c) "A heterogeneous mixture containing only one phase is an impossibility" is incorrect. A heterogeneous mixture is a mixture where the components are not evenly distributed and the mixture has different visible regions or phases. However, it is possible for a heterogeneous mixture to contain only one phase. For example, a mixture of oil and water is heterogeneous but can have only one phase.
To know more about homogeneous visit:
https://brainly.com/question/30587533
#SPJ11
Read the temp what is it?

Answers
how many kilograms of zinc that is 33% pure should be mixed with 12 kilograms of alloy that is 45% pure to make an alloy that is 39% pure zinc
Answers
4.68 kg of zinc, When the oxidation is removed, zinc transforms into a polished steel that is mildly brittle at room temperature. It is the first element in Periodic Table Group 12 of elements.
What is zinc?With the atomic number 30 and the symbol Zn, zinc is a chemical element. When the oxidation is removed, zinc transforms into a shiny-greyish metal that is slightly brittle at normal temperature. It is the first element in the periodic table's group 12 of elements. Kilogram of zinc =12 × 39/100 = 4.68Even though the body only requires minute amounts of zinc, nearly 100 enzymes depend on it to perform essential chemical reactions. It plays a crucial role in the production of DNA, cell proliferation, the synthesis of proteins, the repair of damaged tissue, and the maintenance of a strong immune system.It is definitely best to stay away from this supplement if you have a zinc allergy. Numerous conditions may be helped by zinc, or they may be negatively impacted by it due to improper management.To learn more about Zinc, refer to:
brainly.com/question/1594082
#SPJ4
Is silicon(IV) oxide the same as silicon dioxide
Answers
Answer:
Yes, it is
Explanation:
Silicon (IV) oxide, commonly known as silica is a substance that majorly constitutes SAND. It has the chemical formula, SiO2, which contains a combination of silicon and oxygen.
The IUPAC name of SiO2 is Silicon IV oxide, which can also be referred to as Silicon dioxide because the structure the compound contains two (-di) oxygen atoms. Hence, based on the question, silicon(IV) oxide is the same as silicon dioxide.
(13 POINTS + BRAINLIEST) On a hot and windy day a farmer is cleared all the grasses from a field next to a stream in order to plant some crops. He worked all day removing the grasses, and planting seeds for his next crop. The next day the farmer noticed that half of his field had eroded away. What choice below best describes why the river was able to erode so much of the field?
A.
The wind blew the freshly cleared dirt in the field away.
B.
The river was eroded the ground since the grasses and roots were removed.
C.
Animals dug into the freshly cleared dirt looking for food.
D.
The hot temperatures caused the ground to crack and fall apart.
Answers
Answer:
B, The river was eroded the ground since the grasses and roots were removed.
Explanation: when you have roots in the ground they would soak up the water but because he removed the roots, The water goes where it used to and it eroded the ground.
balance the following skeleton reaction, calculate e o cell , and state whether the reaction is spontaneous. agcl(s) no(g) → ag(s) cl−(aq) no3−(aq) [acidic] balanced reaction: e o cell
Answers
The balanced reaction is: 2AgCl(s) + 2NO(g) → 2Ag(s) + Cl^-(aq) + 2NO3^-(aq). The standard cell potential (Eo cell) can be calculated by summing the standard reduction potentials of the half-reactions involved in the reaction.
How do we calculate Eo cell for the given reaction?To calculate Eo cell, we need to consider the reduction half-reactions for the species involved in the reaction. The reduction half-reactions are:
1. Reduction of Ag+ to Ag: Ag+(aq) + e^- → Ag(s) (Eo = +0.80 V)
2. Reduction of NO to NO3^-: NO3^-(aq) + 2H+(aq) + e^- → NO(g) + 2H2O(l) (Eo = +0.96 V)
By adding these two half-reactions, we can obtain the overall balanced redox reaction. Since Ag+ and NO3^- are spectator ions, they do not participate in the reaction. Thus, the balanced redox reaction becomes:
2AgCl(s) + 2NO(g) → 2Ag(s) + 2Cl^-(aq) + 2NO3^-(aq)
The standard cell potential (Eo cell) is the sum of the reduction potentials of the half-reactions:
Eo cell = Eo reduction of Ag+ to Ag + Eo reduction of NO to NO3^-
= (+0.80 V) + (+0.96 V)
= +1.76 V
Learn more about: balanced reaction
brainly.com/question/14258973
#SPJ11
How does the molecular formula of a compound differ from the empirical formula? Can a compound’s empirical and molecular formulas be the same? Explain.
Answers
NEED HELP ASAP!!! 20 POINTS!!
Which of the following is an exothermic reaction?
A) N2(g) + 2O2(g) → 2NO2(g) ∆H = +66.4 kJ
B) 2C(s) + H2(g) +227.4 → C2H2(g)
C) 4Al(s) + 3O2(g) → 2Al2O3(s) + 3351 kJ
D) N2(g) + O2(g) → 2NO(g) ∆H = +182.6
Answers
Answer:
exo means energy is released when delta h is + it is endo and when energy is on the reactants side that is energy introduced so endo as well so when energy shows up on the product side that is the exo one
so it is C as that E is listed as a product therefore released
Explanation:
which layer of the earth has the lowest density
Answers
Answer:
lithosphere
Explanation:
Earth's interior layers are ordered by density. The densest layer is the solid metal inner core, the mantle is of intermediate density, and the least dense layer is the lithosphere, particularly the continental lithosphere.
The crust is the layer of the earth with the lowest density.
The crust is the rock layer that is the lightest and most buoyant on Earth. 41% of the Earth's surface is covered by continental crust, with a quarter of that covered by ocean floor. The thickness of the continental crust is 20 to 80 kilometers. Four billion years of Earth's history are stored in its rocks.
The crust is the outermost layer of the Earth, and it is only about 5-70 km deep. The mantle is the layer beneath the crust, which is about 2,900 km thick and has a thickness that varies. The core is divided into two sections: the inner core, which is solid, and the outer core, which is liquid. The Earth's surface is made up of several plates, which are continuously changing due to forces within the Earth.
Know more about crust:
https://brainly.com/question/13428623
#SPJ6
1. Write the conversion factor need for each unit conversion.
a. feet →yards
c. yards → rods
e. feet → miles b. years → days
d. days → hours
f. seconds → minute

Answers
a.) 1 foot = 0.33 yard.
b.) 1 year = 365 days
c) 1 yard = 0.18 rod
d) 1 foot= 0.0001893 mile
e) 1 day = 24 hours
f) 60 seconds = 1 minute
Conversion factorsAccording to the international system of measurement, the conversion factor needed for the following unit conversions are as follows:
Feet to yards: 1 foot = 0.33 yard.Yards to rods: 1 yard = 0.18 rodYear to days: 1 year = 365 daysFeet to mile: 1 foot= 0.0001893 mileDays to hours: 1 day = 24 hoursSeconds to minute: 60 seconds = 1 minuteMore on conversion factors can be found here: https://brainly.com/question/20822566
Can a plane mirror ever produce a real image ? Explain
Answers
Answer:
\(\huge\fbox{Answer ☘}\)
Yes,a plane mirror can form a real image. A plane mirror can form a real image only for a virtual object. These converging rays of incidents light after reflection intersect at a point to give a real image.
hope helpful~
Plane mirrors always produce virtual images, because they never focus light into a single converging point.
Hence it does not produce real object or image
As power of the plane mirror is zero, hence, it neither converges nor diverges the rays.
What type of system is impacting weather in Houston?
A) High pressure
B) Low pressure
C) Warm pressure
D) Cold pressure

Answers
Answer:
a) high pressure
that is the answer i got, but wait for a few more answers before you write the answer
It can be difficult to differentiate a system at equilibrium from a system containing a?
Answers
It can be difficult to differentiate a system at equilibrium from a system containing a very slow chemical reaction.
It can be difficult to differentiate a system at equilibrium from a system containing a very slow chemical reaction because the phase that moves slowly needs more time to complete because it may entail numerous other processes.
As an illustration, a reactant would need to diffuse as well as migrate to a certain reaction site before the next change can be implemented, which then immediately creates a product.
It can be challenging to distinguish an equilibrium system from one that has: Depending on the circumstances, a reversible reaction may only take place in one direction and the reverse reaction may not contribute much.
Equilibrium is influenced by the system's temperature, pressure, as well as concentration. When one of these variables changes, the system's equilibrium is upset and it returns to normal itself so that it reaches equilibrium again.
Therefore, It can be difficult to differentiate a system at equilibrium from a system containing a very slow chemical reaction.
To know more about equilibrium
https://brainly.com/question/24317238
#SPJ4
if 4.00 ml m l of vinegar needs 43.5 ml m l of 0.150 m m naoh n a o h to reach the equivalence point in a titration, how many grams of acetic acid are in a 1.30 qt q t sample of this vinegar?
Answers
If 4.00 mL of vinegar needs 43.5 mL of 0.150 m NaOH to reach the equivalence point in a titration, The grams of the acetic acid is 119.9 g.
Given that :
The molarity of NaOH = 0.150 M
The volume of NaOH = 43.5 mL = 0.0435 L
The moles = molarity × volume
= 0.150 × 0.0435
= 0.0065 mol
At equivalence point ,
Moles of CH₃COOH = moles of NaOH
The molarity of the CH₃COOH = moles / volume
= 0.0065 / 0.004
= 1.625 mol
Volume of CH₃COOH = 1.50 × 0.946353
= 1.230 L
The mass of the Acetic acid = 1.625 × 1.230 × 60
= 119.9 g
To learn more about equivalence point here
https://brainly.com/question/29033775
#SPJ4
given that k(eq) = k(sp), calculate concentration of the aqueous cation formed at equilibrium when excess solid agi is placed in water if k(eq) = 8.52 × 10⁻¹⁷.
Answers
The concentration of the aqueous cation formed at equilibrium is 9.23 × 10⁻⁹ M.
Determine the concentrationTo calculate the concentration of the aqueous cation
formed at equilibrium when excess solid AgI is placed in water, we can use the equation for the equilibrium constant, K(eq), which is equal to the product of the concentrations of the products divided by the product of the concentrations of the reactants.
In this case, the equation is: K(eq) = [Ag⁺][I⁻]/[AgI]
Since K(eq) = K(sp), we can substitute the value of K(sp) into the equation: 8.52 × 10⁻¹⁷ = [Ag⁺][I⁻]/[AgI]
We know that the concentration of AgI is in excess, so we can assume that it is essentially constant. Therefore, we can simplify the equation to:
8.52 × 10⁻¹⁷ = [Ag⁺][I⁻]
Since AgI is a 1:1 salt, the concentration of Ag⁺ is equal to the concentration of I⁻.
Therefore, we can substitute [Ag⁺] for [I⁻] and solve for [Ag⁺]:
8.52 × 10⁻¹⁷ = [Ag⁺]² [Ag⁺] = √(8.52 × 10⁻¹⁷) [Ag⁺] = 9.23 × 10⁻⁹ M
Therefore, the concentration of the aqueous cation formed at equilibrium is 9.23 × 10⁻⁹ M.
Learn more about cation at
https://brainly.com/question/28710872
#SPJ11
How many moles of hcl are present in 0. 70 l of a 0. 33 m hcl solution?.
Answers
Answer:
0.231 mol HCl
Explanation:
What we know:
Volume of HCl solution = 0.70 LStrength of HCl solution = 0.33 MHere M stands for molarity. Molarity is the number of moles of the substance present per Litre of solution. So in place of M we can write moles / L
Number of moles of HCl solution = Volume of HCl solution x Strength of HCl solution
= 0.70 L x 0.33 moles / L
= 0.231 mol HCl
0.231 moles of HCl are present in 0.70 L of a 0.33 M HCl solution
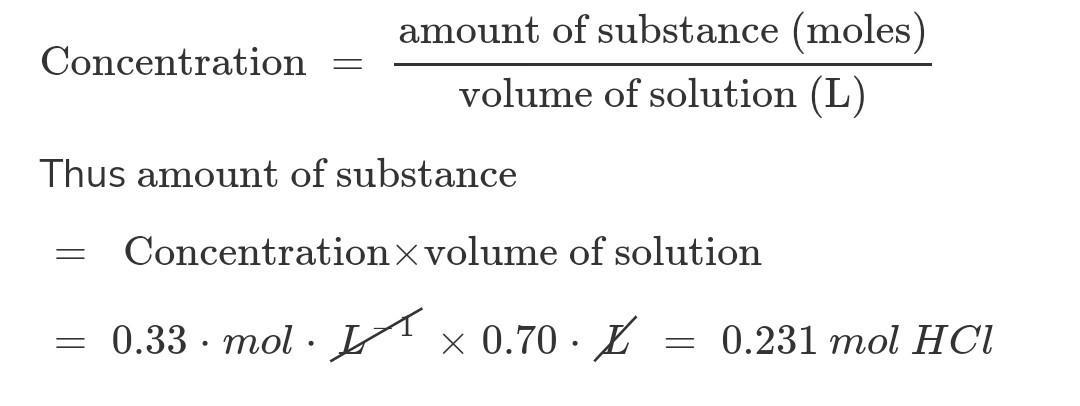
What is the concentration of an aqueous solution that contains 1.5 moles of NaCl in 500 milliliters of this solution? A) 3.5 g. B) 3.5 M. C) 3.5 mL. D) 3.5 mol.
Answers
The concentration of the aqueous solution, or 3.5 M, is the result (B).
To find the concentration of an aqueous solution, we need to know the amount of solute (in moles) and the volume of the solution (in liters).
The amount of solute given in the problem is 1.5 moles of NaCl.
The volume of the solution given is 500 milliliters, which needs to be converted to liters by dividing by 1000: 500 mL ÷ 1000 = 0.5 L.
Now we can use the formula for concentration, which is:
concentration (in M) = moles of solute ÷ volume of solution (in L)
Plugging in the values we have:
concentration (in M) = 1.5 moles ÷ 0.5 L = 3.0 M
So the answer is B) 3.5 M which represents the concentration of the aqueous solution.
To learn more about aqueous solution refer to:
brainly.com/question/26856926
#SPJ4
What is the electron structure of an oxygen atom?
Answers
#CarryOnLearning
For each of the following sets of elements, state whether all of the elements are in the same group, the same period, or neither the same group or the same period.
a. Fe, Ru, Os
b. Rh, Pd, Ag
c. Sn, As, S
d. Se, Te, Po
Answers
Answer:
a. Same group, different period
b. Different group, same period
c. Different group, different period
d. Same group, different period
explain why elements with high ionization energies typically also have high electronegativity values
Answers
Elements with high ionization energies typically also have high electronegativity values.
This is because the strength of the attraction between an atom and the electrons it contains affects both properties. The more strongly an atom attracts its electrons, the higher it's ionization energy and electronegativity.
This is because it requires more energy to remove the electrons from the atom, resulting in higher ionization energy, and it will also take more energy to attract electrons away from another atom, resulting in a higher electronegativity. This means that elements with high ionization energies typically also have high electronegativity values.
For more questions like Electronegativity click the link below:
https://brainly.com/question/490663
#SPJ4