What is the empirical formula mass of c4h6f2? (atomic masses: c = 12 amu, h = 1 amu, f = 19 amu). enter your value as a whole number.
Answers
Molar Mass, Molecular Weight and Elemental Composition Calculator ; Carbon, C · 12.0107, 4, 52.1710 ; Hydrogen, H · 1.00794, 6, 6.5673.
What is Molar Mass,?The mass of a sample of a chemical compound is divided by the quantity of that substance, or the number of moles in the sample, measured in moles, to obtain the compound's molar mass in chemistry.
In contrast to molecular properties, a substance's molar mass is a bulk property. The compound is present in many different forms, each with a different mass as a result of the isotopes. The molar mass is the average of these masses. The molar mass is most frequently calculated using the standard atomic weights and is a function of the relative abundance of the constituent atoms' isotopes on Earth. It is thus a terrestrial average. In order to convert between a substance's mass and its amount, the molar mass is the suitable unit.
To learn more about Molar Mass, from the given link:
https://brainly.com/question/19584119
#SPJ4
Related Questions
Why is the Sun's lifespan primarily based on its initial mass?
Answers
Answer:
Probably because there is more helium to burn. After billions of years there's only gonna be helium so no more of that firey energy.
I think....
Please answer question #4
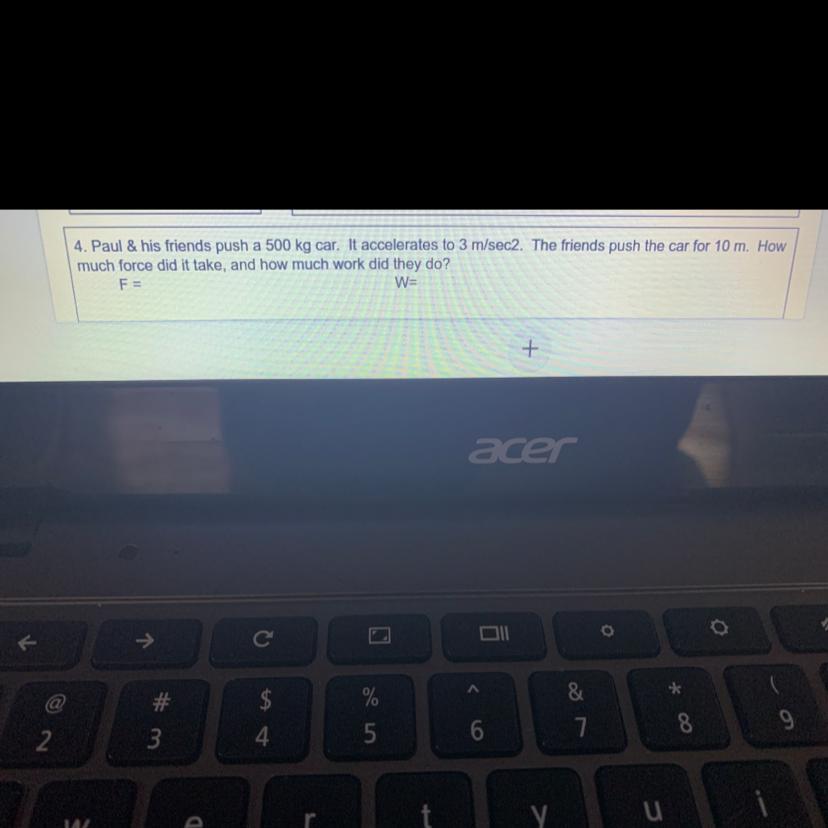
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
.. When water boils, you can see bubbles rising to the surface of
the water. Of what are these bubbles made?
a. air
b. hydrogen and oxygen gas
c. oxygen gas
d. water vapor
e. carbon dioxide gas
Answers
Answer:
Explanation:
It's water vapor. There is enough heat present to get the water to boil but not enough to break it into its chemical components (oxygen and hydrogen), so the answer is D.
How many molecules of NaOH are in 10.0 g of NaOH? *
Answers
The number of molecules in 10.0 gram of NaOH is 15 * 10²².
To solve this question, we need to understand some terms of mole concept,
Mole - It is the amount of substance containing same number of molecules or atoms as there are atoms in 12 gram of carbon-12 isotope.
Molecules - It is group of atoms bonded together, representing the smallest fundamental unit of a chemical compound taking part in chemical reaction.
Molecular weight - The sum of atomic masses of all atoms in molecules.
Avogadro number - It is the number of atoms, ions, electrons, molecules in one mole of substance. It is represented as NA.
NA = 6.0 * 10²³ (approx)
To calculate the number of molecules, we apply the formulae,
no. of molecules = moles * NA
moles = weight / molecular weight
moles = 10.0 / 40
= 0.25
Substituting this value to calculate number of molecules,
no. of molecules = 0.25 * 6.0 * 10²³
= 15 * 10²²
Therefore the number of molecules of in 10.0 g of NaOH is 15 * 10²².
To know more about moles and molecules,
https://brainly.com/question/29367909
In which substance are molecules rigidly arranged?
1) water in a freezer at -2 degrees Celsius
2) water at cup at 22 degrees Celsius
3) water in a pot at 100 degrees Celsius
4) water in a steam engine at 110 degrees Celsius
Answers
Answer:
Water in a freezer at -2 degrees Celsius
50 points - I need this really soon! plz help
The pictured compound has which bond?
A.) C = C and C −C
B.) C = C and C −H
C.) C −Cand C −H
D.) C −C, C −H, and C −O

Answers
Answer:
C.
Explanation:
Butane (C4H10) has 3 C-C bonds in the carbon chain and 10 C-H bonds
Butane (\(C_4H_10\)) has 3 C-C bonds in the carbon chain and 10 C-H bonds
What is alkane?Alkanes are saturated hydrocarbons. This means that their carbon atoms are joined to each other by single bonds.
Based on the diagram, butane is considered to be an alkane. It not only contains single covalent bonds, but also has carbon and hydrogen atoms present in its structure.
When comparing both structures to one another, isobutane is a branched chain, while butane is a linear chain.
Learn more about alkane here:
https://brainly.com/question/4260635
#SPJ2
Use the change of base rule to find the logarithm to four decimal places. log 143.2 O 0.2213 O 4.5186 2.2593 O
0.4771
Answers
Using the change of base rule to find the logarithm to four decimal places. the correct answer is 11.4235.
To find the logarithm of 143.2 using the change of base rule, we can choose any base we prefer. Let's use base 10 and natural logarithm (base e) for this calculation.
First, we'll use the change of base formula, which states that log(base b) x = log(base c) x / log(base c) b. In this case, we'll calculate log(base 10) 143.2.
We'll use the natural logarithm (ln) as our intermediary step. The natural logarithm of 143.2 can be calculated as ln(143.2).
Using a calculator, we find that ln(143.2) is approximately 4.9628.
Next, we need to calculate log(base 10) e, which is the logarithm of e with base 10. Using a calculator, we find log(base 10) e is approximately 0.4343.
Finally, we apply the change of base formula:
log(base 10) 143.2 ≈ ln(143.2) / log(base 10) e
≈ 4.9628 / 0.4343
≈ 11.4235
Rounding to four decimal places, the logarithm of 143.2 using base 10 is approximately 11.4235.
Learn more about logarithm here :-
https://brainly.com/question/30226560
#SPJ11
Which electron configuration represents an atom of chlorine in an excited state?
1) 2-7-7
2)2-7-8
3)2-8-7
4)2-8-8
Answers
Answer:
3
Explanation:
What happens to unstable nuclei? (Select all that apply.)
1. They decay into more stable nuclei.
2. Their mass decreases by 50% with each half-life.
3. They emit radiation.
4. They combine to form more stable nuclei.
Answers
The activities which occurs in unstable nucleus from the above choices are:
They decay into more stable nuclei.Their mass decreases by 50% with each half-life.They emit radiation.They combine to form more stable nuclei.The correct answer choices are all 1, 2, 3 and 4.
How unstable nucleus decay into more stable nucleusIt follows that unstable nucleus usually, frequently and most of the time emit radiation and when this occur, it results to decay of substances.
From the task given above, it is crystal clear that that all the choices given above are happenings which occur when an unstable nucleus undergoes radioactive decay.
So therefore, we can now confirm that the nucleus of unstable atoms does decay into more stable nucleus.
Read more on unstable nucleus:
https://brainly.com/question/7067113
#SPJ1
g which of the following compounds is correctly classified as a tertiary alcohol? selected answer: correcta. 2-methyl-2-butanol answers: correcta. 2-methyl-2-butanol b. 2-methyl-1-butanol c. 3-methyl-1-butanol d. 3-methyl-2-butanol
Answers
The correct answer is 2-methyl-2-butanol. A tertiary alcohol is an alcohol that has three alkyl groups attached to the carbon atom that is bonded to the hydroxyl (-OH) group.
In the case of 2-methyl-2-butanol, the carbon atom bonded to the hydroxyl group is also bonded to three methyl groups, making it a tertiary alcohol.
The other options, 2-methyl-1-butanol, 3-methyl-1-butanol, and 3-methyl-2-butanol, are all classified as primary or secondary alcohols, as they have only one or two alkyl groups attached to the carbon atom bonded to the hydroxyl group. Therefore, the correct answer is 2-methyl-2-butanol.
know more about alkyl groups here
https://brainly.com/question/6846666#
#SPJ11
Please, can anyone confirm that my answers are correct?
Will give brainiest to first correct answer! (No links pls)




Answers
40.0 L of oxygen were collected at 10°C and 758 mm of Hg.
Calculate its volume at STP
Answers
Answer:
38.48 L. Good luck on your test :)
Explanation:
I clicked it and it was right.
How many orbitals exist for s shape
Answers
what is the ratio of effusion rates for the lightest gas, h2, to the heaviest known gas, uf6?
Answers
The ratio of effusion rates for the lightest gas H₂ to the heaviest known gas UF₆ is 13.21 to 1
What is effusion?Effusion is a process by which a gas escapes from its container through a tiny hole into evacuated space.
Rate of effusion ∝ 1/√Ц, (where Ц is molar mass)
Rate H₂ = 1/√ЦH₂
Rate UF₆ = 1/√ЦUF₆
Therefore, Rate H₂/ Rate UF₆ = √ЦH₂/√ЦUF₆
ЦH₂= 2.016 g/mol
ЦUF₆= 352.04 g/mol
Rate H₂ / Rate UF₆ = √352.04/√2.016 = 18.76/1.42
Rate H₂ / Rate UF₆ = 13.21
Therefore, H₂ is lower mass than UF₆. Thus H₂ gas will effuse 13 times more faster than UF₆ because the most probable speed of H₂ molecule is higher; therefore, more molecules escapes per unit time.
learn more about effusion rate: https://brainly.com/question/28371955
#SPJ1
According to the following chemical
reaction
SiO2(s) + 3C(s) + 3 SIC(s) + 2C0(g)
if 3 mol of SiO2 are added to 4 mol of C
How many moles of excess reactant
remain?
Answers
surface tension occurs water molecules form a weak bond with each other allowing a small bug to walk across the surface of a puddle.these bonds are rferred to as
Answers
The bonds when surface tension occurs, water molecules form a weak bond with each other allowing a small bug to walk across the surface of a puddle. are referred to as hydrogen bonds.
Surface tension is the energy, often denoted as γ, necessary to increase the surface area of a liquid by a unit of area. Surface tension is a result of the cohesive forces that arise between the liquid's atoms and molecules. It's a property of liquids that makes them behave like an elastic sheet or membrane on their surface.
Hydrogen bonds are a type of chemical bond that occurs between a hydrogen atom bonded to an atom such as oxygen, nitrogen, or fluorine and another atom or electronegative group in a different molecule or chemical group.
The hydrogen bond is a type of dipole-dipole interaction that occurs when an electronegative atom or molecule attracts the positively charged hydrogen atom of another molecule or group. The hydrogen bond is a result of the difference in electronegativity between the hydrogen and the electronegative atom or group.
Learn more about surface tension https://brainly.com/question/571207
#SPJ11
Using Boyle’s Law, calculate the new volume of the weather balloon at a higher altitude. Assume the volume of the balloon at ground level is 10.0 m3 where the atmospheric pressure is 1.00 atm. What will be its new volume at an altitude of 15 km where the atmospheric pressure is 0.350 atm?
Answers
Answer:
Explanation:
V1 = 10 m^3
P1 = 1.00 atmospheres
V2 = ?
P2 = 0.35
Formula
V1 * P1 = V2 * P2
Solution
10 * 1 = x * 0.35 Combine the left and divide both sides by 0.35
10/0.35 = x
x = 28.57 m^3
The volume of a beaker is 250 cm Three beakers can hold
mL water.
Help me pls.
Answers
Answer:750 mL
Explanation:
volume=3x250 cm^3 = 750 cm^3
750 cm^3 x 1mL/1cm^3 =750 mL

If the volume of a beaker is 250 cm. The amount of water that three beakers can hold is equal to 750 mL.
What is Volume?Volume may be defined as the amount of space that is significantly occupied by a three-dimensional figure or object. It is measured in cubic units.
According to the question,
The volume of a beaker is = 250 cm.
It is known that 1 milliliter is exactly the same as 1 cm.
∴ The amount of water three beakers can hold = 250 × 3 = 750 mL.
Therefore, if the volume of a beaker is 250 cm. The amount of water that three beakers can hold is equal to 750 mL.
To learn more about Volume, refer to the link:
https://brainly.com/question/1972490
#SPJ2
explain why the ability of plp to catalyze an amino acid transformation is greatly reduced if a plp-requiring enzymatic reaction is carried out at a ph at which the pyridine nitrogen is not protonated.
Answers
If the pH of the environment is such that the pyridine nitrogen of PLP is not protonated, the ability of PLP to catalyze amino acid transformations is significantly reduced. The protonation of the pyridine nitrogen is necessary for the formation of Schiff base intermediates and subsequent enzyme-catalyzed reactions involving PLP.
Pyridoxal 5'-phosphate (PLP) is a coenzyme that plays a crucial role in many enzymatic reactions involving amino acids. PLP acts as a cofactor, facilitating the transfer of functional groups during these transformations. The ability of PLP to catalyze amino acid transformations relies on its protonated state, which is influenced by the pH of the environment.
At physiological pH, the pyridine nitrogen atom of PLP is protonated, meaning it carries a positive charge. This protonation is essential for PLP's interaction with substrates and the subsequent catalytic activity. The positively charged pyridine nitrogen allows PLP to form Schiff base intermediates with amino acids, forming a covalent bond between the substrate and PLP.
However, if a PLP-requiring enzymatic reaction is carried out at a pH where the pyridine nitrogen is not protonated (i.e., under alkaline conditions or high pH), the ability of PLP to catalyze amino acid transformations is greatly reduced. The loss of the positive charge on the pyridine nitrogen disrupts the formation of Schiff base intermediates, thereby inhibiting the catalytic activity of PLP-dependent enzymes.
The protonation state of the pyridine nitrogen is crucial because it enables the nucleophilic attack by the substrate on the carbonyl carbon of PLP, leading to the formation of a transient Schiff base intermediate. This intermediate then undergoes various chemical transformations, such as decarboxylation, transamination, racemization, or other reactions involved in amino acid metabolism.
In summary, if the pH of the environment is such that the pyridine nitrogen of PLP is not protonated, the ability of PLP to catalyze amino acid transformations is significantly reduced. The protonation of the pyridine nitrogen is necessary for the formation of Schiff base intermediates and subsequent enzyme-catalyzed reactions involving PLP.
learn more about pyridine nitrogen here
https://brainly.com/question/30545214
#SPJ11
a ketohexose is reduced with nabh4 in ch3oh to form a mixture of d-galactitol and d-talitol. what is the structure of the ketohexose? draw your answer as a fischer projection.
Answers
The starting ketohexose must be a hexose that contains both galactose and talose as possible constituents. This indicates that the ketohexose is most likely D-tagatose, which has a ketone functional group and six carbon atoms. The Fischer projection of D-tagatose would show the arrangement of its six carbon atoms in a straight chain with the ketone group on the second carbon atom.
To determine the structure of the ketohexose that yields a mixture of d-galactitol and d-talitol when reduced with NaBH4 in CH3OH, we need to analyze the products. Both d-galactitol and d-talitol are sugar alcohols derived from hexoses. D-galactitol is derived from D-galactose, while D-talitol is derived from D-talose. Therefore, When a ketohexose is reduced with NaBH4 in CH3OH to form a mixture of D-galactitol and D-talitol, the ketohexose in question is D-tagatose. In its Fischer projection, the structure of D-tagatose is as follows:
CHO
|
C(OH)H
|
C(OH)H
|
C(OH)H
|
C(OH)H
|
CH2OH
To convert it into the Fischer projection of D-galactitol, you need to change the top carbonyl (C=O) group to an alcohol (C-OH) group. Likewise, you can obtain D-talitol's Fischer projection by changing the C=O group and inverting the 2nd hydroxyl group's orientation.
To know more about ketohexose visit:
https://brainly.com/question/31114760
#SPJ11
Sodium will do an ionic bond with
Answers
Answer:
Chorine
Explanation:
It's a classic, sodium chloride, table salt, which is an ionic bond.
PLS GIVE BRAINLIEST
The melting point of this substance is __________________.
Put a number in the answer.

Answers
The melting point of the substance is 12.5 degrees Celsius
What is melting pointMelting point is the temperature at which a solid substance changes state from solid to liquid at atmospheric pressure.
At the melting point, the solid and liquid phases of the substance exist in equilibrium, and any further increase in temperature will cause the substance to completely melt into a liquid.
The melting point is a characteristic physical property of a substance and can be used to identify and purify a substance. The melting point of a substance is usually reported in degrees Celsius (°C) or Kelvin (K).
Learn more about melting point at
https://brainly.com/question/40140
#SPJ1
How does what you learned in this investigation help you explain why chefs measure the amount of ingredients they need before preparing foods?
Answers
Chefs measure the number of ingredients they need before preparing foods for accuracy, consistency, and balancing flavors.
Measurements ensure accuracy and consistency in recipes. Cooking is a precise process, and precise measurements of ingredients are crucial for achieving the desired taste, texture, and overall outcome of a dish. By measuring ingredients, chefs can replicate their recipes consistently, ensuring that each dish turns out as intended.
Certain ingredients, such as spices, seasonings, and acids, can greatly impact the taste of a dish. By carefully measuring these ingredients, chefs can maintain a precise balance of flavors.
Learn more about accuracy, here:
https://brainly.com/question/13099041
#SPJ1
The chart below shows the major types of minerals mined in the United States and Australia.
Country Major types of minerals
United States coal, iron, silver, copper, oil
Australia iron, oil, uranium, silver, lead, zinc, bauxite, coal, copper, gold
Based on the chart, what percent of the major types of minerals mined in Australia are also mined in the United States?
A 30%
B 50%
C 70%
D 90%
Answers
Answer:
I think it is b 50%
1. What class of drugs are being investigated in this study, and how do they get into our waterways? 2. What is a C-start and why is it important for larval fish survival? 3. What hypotheses are being tested in this investigation? 4. Briefly describe what the researchers found when they exposed larval fathead minnows to levels of antidepressants found in our waterways.
Answers
The effects of exposure were more pronounced in fish that had been raised in a less stressful environment, suggesting that environmental conditions can influence the impact of exposure to antidepressants.
1. The class of drugs being investigated in this study is antidepressants. They enter our waterways through excretion by individuals taking the medication, and disposal of unused medication into toilets or sinks that are connected to wastewater treatment plants.
2. C-start is an evasive maneuver that young fish use when they perceive a predator. This is important for larval fish survival because it helps them to avoid being eaten by predators.
3. In this investigation, researchers are testing two hypotheses. The first is that exposure to low levels of antidepressants can affect larval fathead minnows' behavior, and the second is that the effects of exposure will be more pronounced in fish that have been raised in a less stressful environment.
4. The researchers found that exposure to antidepressants at levels found in waterways can have a significant impact on the behavior of larval fathead minnows. Specifically, they found that the fish exposed to antidepressants were less likely to respond to the presence of predators, which could increase their risk of being eaten.
They also found that the effects of exposure were more pronounced in fish that had been raised in a less stressful environment, suggesting that environmental conditions can influence the impact of exposure to antidepressants.
To learn more about antidepressants,
https://brainly.com/question/28209828
#SPJ4
During a chemical reaction, a fixed amount of iron combines completely with a fixed amount of oxygen to form iron oxide. Which statement best describes the mass of the iron oxide that is produced?
Answers
Explanation:
The mass of the iron oxide produced is the mass of the fixed amount of oxygen and fixed iron that reacted together.
This is predicated on the law of conservation of mass.
The law of conservation of mass states that "in a chemical reaction, matter is neither created nor destroyed, but atoms are rearranged".
The mass of the product is found by summing up the mass of the reactants.
So if we add the mass of oxygen and iron together, we get the mass of iron oxide that will be produced.
Answer:
help JK answer is The mass is equal to the mass of the iron plus the mass of the oxygen.
Explanation:
is Sulfuric Acid soluble if placed in water
Answers
Answer: Yes, sulfuric acid is highly soluble in water.
Explanation:
In an experiment, how many variables should you change at a time? And why?
Answers
Answer:
it should test one variable at a time.
Explanation: because you will not be able to tell which variable is responsible for the observed results.
A solution with ph 4 has __________ the h concentration of a solution with ph 8.
Answers
A solution with ph 4 has 10000 times the h concentration of a solution with ph 8.
the solution, in chemistry, is a homogeneous mixture of two or more substances whose relative amounts can be varied continuously up to the so-called solubility limit. The term solution is usually applied to the liquid state of aggregation, but gaseous and solid solutions are also possible. Hypotonic, isotonic, and hypertonic solutions (tonic).
A solution is a homogeneous mixture of two or more components with particle sizes less than 1 nm. Common examples of solutions are the sugar in water and salt in water, soda water, etc. In solution, all components appear as a single phase.
Learn more about solutions here
https://brainly.com/question/25326161
#SPJ4
Consider the following potential for two inert gas (Xe) atoms at separation R : U=λe −R/rho
− R 6
A
(a) Calculate the potential energy of the two atoms at equilibrium separation R 0
. Express your answer in terms of an exponential function of (R 0
/rho). (The answer should be in the form: U= (factor) e −R 0
/rho
, and the factor should be determined. (b) If the equilibrium separation R 0
=12rho, find the equilibrium potential energy of the two atoms in terms of λ. (c) Now consider a Xe crystal with N atoms and only nearest neighbor interactions. Find the total interaction energy in units of eV/ atom assuming λ=4156eV and R 0
/rho=12
Answers
The total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Given Potential for two inert gas (Xe) atoms at separation R :
U=λe^(-R/rho)-R^6/a^6
a) To calculate the potential energy of the two atoms at equilibrium separation R_0,
we have to put dU/dR = 0λ e^(-R_0/rho) = (6R_0^6)/(a^6)λ e^(-R_0/rho) = (6(12rho)^6)/(a^6)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12)
The potential energy can be expressed as, U=λe^(-R_0/rho) = ((6(12rho)^6)/(a^6)) * e^(12) * e^(-12rho/rho)= ((6*12^6)/a^6) * e^(-11rho)
b) Given R_0 = 12rho, λ = (6(12rho)^6)/(a^6) * e^(12)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Potential energy U = λe^(-R_0/rho) = (6 * 12^6)/(a^6) * e^(-11rho)c)
The total interaction energy in units of eV/ atom assuming λ = 4156 eV and R_0/rho = 12
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Total energy (U) = (N/2)U = (N/2)λe^(-R_0/rho) = (N/2)(6 * 12^6)/(a^6) * e^(-11rho) = 150N eV/atom.
Therefore, the total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Learn more about energy with the given link,
https://brainly.com/question/2003548
#SPJ11