what is the agriculture thallium?
Answers
Answer:is a trace metal of severe toxicity. Its health concerns via consumption of contaminated vegetables have often been overlooked or underestimated
Explanation: Read it carefully and it explains
Related Questions
________ is dissolved in water to make the solution sold commercially as rubbing alcohol.
Answers
Answer:
Isopropyl alcohol or Propan-2-ol
Explanation:
Hope it helps!
An atom moving at its root mean square velocity at 100. °c has a wavelength of. Which atom is it? assume that the atom is the most abundant isotope of an element.
Answers
The atom that moves at its rms velocity at 100°C with a wavelength of 2.31 * 10 m is : SULPHUR ( s )
Determine the molar mass of the atomTo determine the atom we will have to determine the molar mass of the atom
Applying De Broglie equation
λ = h / mv
Vrms = \(\sqrt{\frac{3RT}{M} }\) ---- ( 1 )
Where : λ = 2.31 * 10⁻¹¹, R = 8.314 J / k.mol, T = 373 K, h = 6.626 * 10⁻³⁴ J.s
From equation ( 1 )
M = ( h² Ua ) / 3RT*λ² --- ( 2 )
where : Ua ( mass of an atom ) = 6.022 * 10²³, h = 6.626 * 10⁻³⁴, R = 8.314 J / k.mol, λ = 2.31 * 10⁻¹¹, T = 373 K
Insert values into equation ( 2 )
M ( molar mass ) = 32 g/mol
Sulphur has a molar mass of 32 g/mol therefore the atom is sulphur.
Hence we can conclude that The atom that moves at its rms velocity at 100°C with a wavelength of 2.31 * 10 m is : SULPHUR ( s ).
Learn more about sulphur : https://brainly.com/question/26328290
40 points/ brainliest
the first step in planning effective writing is to consider the _____.
main idea
audience
style
purpose
Answers
The first step in planning effective writing is to consider the main idea.
Considering the main idea or topic that will be discussed will help the writer to stay focused and organized throughout the writing process.
Next, the writer needs to consider the audience that will be reading their writing. Knowing who the audience is will help the writer to tailor their writing style and language to better connect with the readers.
Style is another important aspect to consider when planning writing. The style of writing can greatly impact how the message is received by the audience. The writer must choose the appropriate tone, structure, and language to convey their message effectively.
Finally, the writer needs to consider the purpose of their writing. This will help them to determine the goals and objectives of their writing and how to structure the content to achieve those goals.
In conclusion, by considering the main idea, audience, style, and purpose, the writer can better plan and execute effective writing that effectively conveys the intended message to the reader. This is why these elements are crucial when it comes to planning effective writing.
Learn more about writing here: https://brainly.com/question/31119902
#SPJ11
Relative atomic mass of an atom is 32. What element is this?
Answers
Answer:
Tungsten
Explanation:
What is the charge of the central metal ion in Ca3[Fe(CN)6]2?A) 3+B) 6+C) 2+D) 1+E) 0
Answers
The charge of the central metal ion (Fe) in Ca\(_{3}\)[Fe(CN)\(^{6}\)]\(^{2}\) is 3+. The correct answer is A) 3+.
To determine the charge of the central metal ion (Fe) in Ca\(_{3}\)[Fe(CN)\(^{6}\)]\(^{2}\), we need to analyze the overall charge of the compound. In Ca\(_{3}\)[Fe(CN)\(^{6}\)]\(^{2}\), there are three Ca2+ ions (3 x 2+ = 6+) and two [Fe(CN)\(^{6}\)] complex ions. To balance the charge, the two [Fe(CN)\(^{6}\)] complex ions must have a combined charge of 6-. Therefore, each [Fe(CN)\(^{6}\)] complex ion has a charge of 3-.
Within the [Fe(CN)\(^{6}\)] complex ion, each CN- ligand carries a charge of 1-. Since there are six CN- ligands, the total negative charge from the ligands is 6-. To achieve the overall charge of 3- for the complex ion, the central metal ion (Fe) must have a charge of 3+. The correct answer is A) 3+.
More on charge: https://brainly.com/question/28304632
#SPJ11
Can someone tell me if these are right? I am supposed to be decoding this for my escape room but it seems to be wrong.
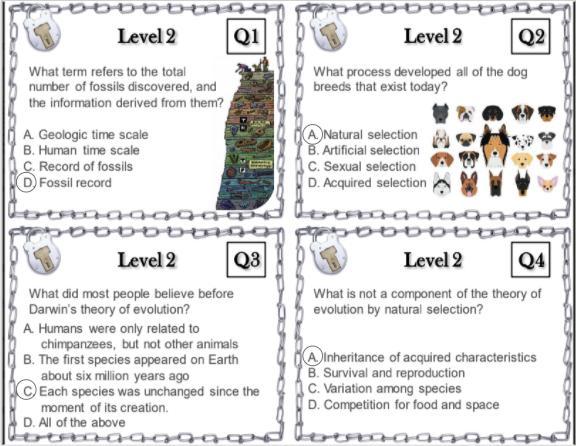
Answers
Answer:
Q1 is A.
Q2 is right
Q3 is D.....I dont
really know about this one
Q4 is right
what is the maximum amount of strong base that can be added to a buffer made by the mixing of 0.35 mol sodium hydrogen carbonate with 0.50 mol sodium carbonate? select one: 0.35 mol 0.35 mol 0.70 mol 1.00 mol
Answers
The maximum amount of strong base that can be added to the buffer without significantly changing the pH is 0.35 mol.
Sodium carbonate is a white, odorless powder that is commonly used in various applications such as in the manufacture of glass, soaps and detergents, water softening, and as a food additive. It is an ionic compound made up of sodium cations (Na+) and carbonate anions ( 2-). It is also known as washing soda, soda ash, or sal soda.
According to given data, Number of moles of NaH = 0.35 mol. To calculate the initial pH of the buffer, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where pKa is the acid dissociation constant of the weak acid in the buffer (in this case, carbonic acid, ), [A-] is the concentration of the conjugate base (in this case, bicarbonate ion, H-), and [HA] is the concentration of the weak acid (in this case, carbonic acid, ).
The pKa of carbonic acid is 6.35, and the concentrations of H- and can be calculated using the initial amounts of NaH and Na2and the stoichiometry of the reaction:
[H-] = [NaH] = 0.35 mol / (0.35 mol + 0.50 mol) = 0.411 M
[NaH] - [H-] = 0.35 M - 0.411 M = -0.061 M
substituting these values into the Henderson-Hasselbalch equation, we get:
pH = 6.35 + log(0.411 / (-0.061))
pH = 9.17
To know more about buffer solution here
https://brainly.com/question/13049786
#SPJ4
How does a carbon atom in carbon dioxide become a hydrocarbon and organic compound before becoming carbon dioxide again?
Answers
Plants use sunlight and cellular respiration to transform carbon dioxide into the hydrocarbon molecule glucose, which is then ingested by the plants.
How does photosynthesis work?During photosynthesis, oxygen and glucose are created from water and carbon dioxide; the plant uses the glucose and produces oxygen as a waste product.
In the process of cellular respiration, oxygen & glucose are transformed into carbon dioxide and water. Carbon dioxide, water, & ATP, which would be converted into energy, are the process' byproducts.
What makes photosynthesis so crucial?The main purpose of photosynthetic is to transform chemical energy into solar energy, which is then stored for future use. This mechanism primarily provides energy to the planet's life systems.
Learn more about photosynthesis, here:
brainly.com/question/9175642
#SPJ1
What general statement can you make about the global distribution of smartphone minerals?
Answers
Smartphones are genuinely global devices because to their capacity for global communication and their mineral list of different nationalities.
Typically created by inorganic processes, a mineral is a naturally occurring homogenous solid having a specific chemical composition with a highly organised atomic arrangement. About 100 of the known mineral species—the so-called rock-forming minerals—make up the majority of the known mineral species, which number in the thousands.
Smartphones are genuinely global devices because to their capacity for global communication and their ingredient list of different nationalities. However, because minerals are obtained from every corner of the world, the threat of a supply interruption is more pressing than ever.
To know more about mineral, here:
https://brainly.com/question/18078524
#SPJ1
What is the pH of a 3.96 mol/L solution of a weak acid that has an acid dissociation constant of 1.4×10
−5
? Give your final answer to 2 decimal place.
Answers
For the concentration of 3.96 mol/L solution of a weak acid with dissociation constant 1.4x10^-5 pH is 4.256.
To determine the pH of a solution of a weak acid, we need to use the acid dissociation constant (Ka) and the concentration of the acid.
The equation for the dissociation of the weak acid, HA, is as follows:
HA ⇌ H+ + A-
The acid dissociation constant (Ka) is given as 1.4×\(10^-5\), which represents the equilibrium constant for the dissociation reaction. It can be written as follows:
Ka = [H+][A-] / [HA]
Since the concentration of the weak acid, [HA], is 3.96\(\frac{mol}{L}\), and we assume that initially, before any dissociation, [H+] = 0, and [A-] = 0, we can rearrange the equation to solve for [H+]:
Ka = [H+][A-] / [HA]
1.4×\(10^-5\) = [H+][0] / 3.96
Simplifying the equation:
[H+] = (1.4×\(10^-5\)) * (3.96)
[H+] = 5.544×\(10^-5\)\(\frac{mol}{L}\)
The concentration of H+ ions is 5.544x\(10^-5\) \(\frac{mol}{L}\) To find the pH, we can take the negative logarithm (base 10) of the H+ concentration:
pH = -log[H+]
pH = -log(5.544×\(10^-5\)))
pH ≈ 4.256
Therefore, the pH of the 3.96 \(\frac{mol}{L}\) solution of the weak acid with an acid dissociation constant of 1.4×\(10^-5\) is approximately 4.256. Learn more about pH here: https://brainly.com/question/2288405 #SPJ11
A substance such as NaCl dissolves in water because the strong ionic forces that exist in solid NaCl can be overcome by, and replaced by, forces between ________ and the ions.
Answers
A substance such as NaCl dissolves in water because the strong ionic forces that exist in solid NaCl can be overcome by, and replaced by, forces between water molecules and the ions.
When NaCl is dissolved in water, the polar water molecules surround the positively charged sodium ions (Na+) and the negatively charged chloride ions (Cl-) through a process called hydration or solvation. The partially positive hydrogen atoms of water molecules are attracted to the chloride ions, while the partially negative oxygen atoms are attracted to the sodium ions. These attractions, known as ion-dipole forces, are strong enough to overcome the ionic forces holding the NaCl lattice together. As a result, the water molecules surround and separate the Na+ and Cl- ions, effectively dissolving the NaCl compound. This process occurs due to the polar nature of water, which allows it to interact with the charged ions.
To learn more about NaCI click here: brainly.com/question/30766751
#SPJ11
Phosphorus-32 has a half-life of 14.0 days d a y s . Starting with 2.00 g g of 32P 32 P , how many grams will remain after 98.0 days d a y s ?
Answers
The mass (in grams) of the Phosphorus-32 that will remain after 98 days, given that it has a half-life of 14 days is 0.016 g
How do I determine the amoun remaining after 98 days?To determine the amount that will remain after 98 days, we shall first obtain the number of half lives that has passed. This is shown below:
Half-life (t½) = 14 daysTime (t) = 98 daysNumber of half-lives (n) =?n = t / t½
n = 98 / 14
n = 7
Now, we shall determine the amount remaining after 98 days. Details below:
Number of half-lives (n) = 7Original amount (N₀) = 2.00 gAmount remaining (N) = ?N = N₀ / 2ⁿ
N = 2 / 2⁷
N = 2/ 128
N = 0.016 g
Thus, we can conclude that the amount that would remain after 98 days is 0.016 g
Learn more about amount remaining:
https://brainly.com/question/28440920
#SPJ1
Can someone pls help me with a paragraph of what endangered wildlife means ?
Answers
Answer:
Endangered wildlife means that something is at risk of extinction.
Explanation:
Hope I helped
the electron dot formula for o2 molecule shows: a. a single covalent bond b. a double covalent bond c. an ionic bond d. a total of 16 electron dots
Answers
The electron dot formula for the O2 molecule shows a double covalent bond, which means that each oxygen atom shares two electrons with the other oxygen atom.
a. A double covalent bond.
This results in a total of 16 electron dots for the O2 molecule, with 8 dots around each oxygen atom representing the 8 valence electrons they each possess. Therefore, the correct answer to your question is (b) a double covalent bond and (d) a total of 16 electron dots. I hope this helps! Let me know if you have any more questions.
1. Oxygen has 6 valence electrons in its outer shell.
2. To achieve a stable electron configuration, each oxygen atom needs to share two electrons with another oxygen atom.
3. When two oxygen atoms share two pairs of electrons, they form a double covalent bond (O=O).
4. The electron dot formula for the O2 molecule represents this double covalent bond.
So, the correct answer is a double covalent bond.
To know more about covalent bond visit:-
https://brainly.com/question/19382448
#SPJ11
What is a total eclipse?
A) When the Sun partially blocks out
the moon.
B) When the moon partially blocks
out the Sun.
C) When the moon blocks out the
Sun entirely.
D) When the Sun blocks out the
moon entirely.
Answers
Hope it helps ~
The hydrate compound c u s o 4 ⋅ 5 h 2 o is blue in color, while the dehydrated form of copper sulfate c u s o 4 is white. What procedure do you think could be used to convert the pentahydrate form to the dehydrated form?.
Answers
Answer:
Evaporate a solution of CuSO4⋅5H2O to dryness
Explanation:
To dehydrated you have to get rid of the water and water evaporates so you have to evaporate the solution.
Procedure that could be used to convert the pentahydrate form to the dehydrated form is to evaporate a solution of CuSO₄⋅5H₂O to dryness.
Copper(II) sulfate pentahydrate (CuSO₄·5H₂O) form aqueous solutions of bright blue.
After the heating, dehydrated form copper(II) sulfate is formed.
Salt copper(II) sulfate has white color.
Adding a water in white copper(II) sulfate would make solution blue again.
Hydrate is a substance that contains water or its constituent elements.
M(CuSO₄·5H₂O) = 249.5 g/mol; molar mass of copper sulphate pentahydrate
Evaporation occurs when the molecules at the surface of a liquid gain enough energy to overcome the force of attraction of other molecules in the liquid phase.
More about CuSO₄⋅5H₂O: brainly.com/question/8895853
#SPJ4
what elements of the periodic table make up the transmission on a car
Answers
Answer:
Are you asking for all of them? Some of the basic ones are alluminum and carbon.
Bismuth. The pearl pigment of bismuth oxychloride was used in automobile paint for a short time, between 1998 and 2000. ...
Calcium. Calcium carbide is an important mineral in the production of steel.
Carbon. ...
Chlorine. ...
Gold. ...
Helium. ...
Magnesium.
How can knowing that something is
“matter" help you to identify a substance?
Answers
Answer:
knowing that something is a matter help you identify whether a substance is either solid, liquid or gas
Explanation:
knowing that a certain substance falls under the three groups is important. solid has a fixed volume, shape and mass, but cannot be compressed and cannot flow, liquid has a fixed volume but no fixed shape, cannot be compressed but can glow, gas has no fixed shape, no fixed volume, can be compressed and can flow
The pH of a 0.0001M (10-4 M) HCl solution is _______; The pH of a sample of milk is 6.5, while a sample of soft drink has a pH of 4.5. The hydrogen ion concentrations ([H ]) of the two samples are _______________________.
Answers
The pH of a 0.0001M (10-4 M) HCl solution is 4 ; The pH of a sample of milk is 6.5, while a sample of soft drink has a pH of 4.5. The hydrogen ion concentrations ([H ]) of the two samples are 3.16 x \(10^{(-7)\) mol/L, 3.16 x \(10^{(-5)\) mol/L.
To determine the pH of a solution, we can use the equation:
pH = -log[H+]
1. For a 0.0001M (\(10^{-4\) M) HCl solution:
pH = -log(0.0001)
pH = -(-4)
pH = 4
Therefore, the pH of the 0.0001M HCl solution is 4.
2. The pH of milk is 6.5, we can find the hydrogen ion concentration ([H+]) using the inverse of the pH equation:
[H+] = \(10^{(-pH)\)
[H+] = \(10^{(-6.5)\)
[H+] = 3.16 x \(10^{(-7)\) mol/L
Therefore, the hydrogen ion concentration of the milk sample is 3.16 x \(10^{(-7)\) mol/L.
3. Soft drink with a pH of 4.5:
[H+] =\(10^{(-pH)\)
[H+] = \(10^{(-4.5)\)
[H+] = 3.16 x \(10^{(-5)\) mol/L
Therefore, the hydrogen ion concentration of the soft drink sample is 3.16 x \(10^{(-5)\) mol/L.
To know more about concentrations refer here
https://brainly.com/question/3045247#
#SPJ11
A student is studying a sample of neon in a container with a moveable piston (this means the container can change in size). If the sample in the container is initially at a pressure of 757.2 torr when the container has a volume of 81.4 mL, what is the pressure of the gas when the piston is moved so that the volume of the container becomes 132.5 mL? Round your answer to the nearest 0.01 and include units!
Answers
The pressure of the gas in the container when the volume is 132.5 mL is 465.54 torr (rounded to the nearest 0.01) with units of torr.
To solve this question, we can use the ideal gas law equation, PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the universal gas constant, and T is the temperature. Since the sample of neon is at a constant temperature and the number of moles of gas is constant, we can use the equation P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Using the given values, we can write:
P1V1 = P2V2
(757.2 torr) x (81.4 mL) = P2 x (132.5 mL)
Solving for P2, we get:
P2 = (757.2 torr x 81.4 mL) / 132.5 mL
P2 = 465.54 torr
For more such questions on pressure
https://brainly.com/question/17426370
#SPJ11
the balanced equation for the complete combustion of c5h12 will give which of these product(s)? group of answer choices c5h12o5 only 10co 12h2o co2 only 5co2 6h2o 5co2 5h2o
Answers
The balanced equation for the complete combustion of C₅H₁₂ is:
C₅H₁₂ + 8O₂ → 5CO₂ + 6H₂O
Therefore, the product(s) obtained from the complete combustion of C₅H₁₂ are 5CO₂ and 6H₂O. This means that for every molecule of C₅H₁₂ that undergoes combustion, it produces 5 molecules of carbon dioxide (CO₂) and 6 molecules of water (H₂O).
The equation shows that C₅H₁₂, which is a hydrocarbon known as pentane, reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). This is a typical example of a hydrocarbon combustion reaction. The carbon atoms in the hydrocarbon combine with oxygen to form carbon dioxide, while the hydrogen atoms combine with oxygen to form water.
It is important to balance the equation to ensure that the number of atoms of each element is the same on both sides of the equation, following the law of conservation of mass. In this balanced equation, we see that 5 carbon dioxide (CO₂) molecules and 6 water (H₂O) molecules are produced for every molecule of C₅H₁₂ that reacts.
To learn more about complete combustion, here
https://brainly.com/question/14177748
#SPJ4
regions of space around the nucleus of an atom that can be occupied by one or two electrons with identical energy are called
Answers
The regions of space around the nucleus of an atom that can be occupied by one or two electrons with identical energy are called orbitals.
What are the orbitals of the nucleus of an atom?An orbital is a region of space around the nucleus of an atom where electrons are likely to be found. The orbitals of the nucleus of an atom are the shells in which the electrons are found.
The different orbitals have different shapes and energies, and the electrons in different orbitals interact with each other in different ways.
The electrons in the different orbitals interact with each other through a variety of processes, including electrostatic attraction, exchange forces, and spin-orbit coupling. These interactions can lead to changes in the electron’s energy levels, which can affect the properties of the orbital b.
Learn more about the orbitals of an atom:
https://brainly.com/question/26000020
#SPJ4
Write a 200 word summary paragraph discussing this experiment and the results. Use the following questions and topics to help guide the content of your paragraph.
What happened to the cabbage indicator when breath was bubbled into the water? Why?
What happened to the cabbage indicator in the club or clear soda? Why?
Explain the connection between your observations and data and the pH of the oceans.
Give at least one example from real life where the principles demonstrated in this lab are evident.
Answers
This experiment presents the connection between CO2 emissions, ocean acidification, and the significance of pH management in various circumstances in a straightforward yet powerful way.
Why does adding baking soda cause red cabbage to turn blue instead of purple?
A substance known as anthocyanin can be found in red cabbage. This's color varies according to how acidic its surroundings are. It turns pink or red in the presence of an acid, purple when neutral, and blue or green when combined with an alkaline material.
Anthocyanin, a water-soluble pigment found in red cabbage, can change color when combined with an acid or a basic. The color changes from bluish-green to red in alkaline (basic) surroundings with pH levels above 7, and from acidic to basic conditions with pH levels below 7.
To learn more about pH use:
https://brainly.com/question/172153
#SPJ1
draw one possible dipeptide that is formed between alanine and leucine, as the zwitterion.
Answers
To form a dipeptide between Alanine and Leucine, we have to join the carboxyl group (COOH) of Alanine with the amino group (NH₂) of Leucine via a peptide bond. The resulting molecule will have a zwitterionic form. The zwitterionic form of the dipeptide will have both a positive and a negative charge.
A dipeptide is a molecule made up of two amino acid residues joined together via a peptide bond. A peptide bond is a bond between the amino group (NH₂) of one amino acid and the carboxyl group (COOH) of another amino acid. Amino acids are the building blocks of proteins. Alanine and Leucine are two of the twenty common amino acids found in nature.
A zwitterion is a molecule that has a positive charge on one part of the molecule and a negative charge on another part of the molecule. Zwitterions are electrically neutral overall. They are formed when a molecule that has both acidic and basic functional groups is dissolved in a solvent. The acidic and basic groups react with each other to form a neutral molecule that has both positive and negative charges. The zwitterionic form of an amino acid is the form that is found in proteins.
The chemical formula for Alanine is C₃H₇NO₂, and the chemical formula for Leucine is C₆H₁₃NO₂. To form a dipeptide between Alanine and Leucine, we have to join the carboxyl group (COOH) of Alanine with the amino group (NH₂) of Leucine via a peptide bond. The resulting molecule will have a zwitterionic form. The zwitterionic form of the dipeptide will have both a positive and a negative charge.
To know more about dipeptide, refer
https://brainly.com/question/31524411
#SPJ11
Balance the following equations by inserting the proper coefficients.
Answers
The question is incomplete, the complete question is;
Balance the following equations by inserting the proper coefficients.
CH4 + O2 ---------------> CO2 +H2O
CaCl2 + AgNO3 ----->Ca(NO3)2 +AgCl
C2H6O + O2----------->CO2 +H2O
Answer:
CH4 + 2O2 ---------------> CO2 +2H2O
2AgNO3 + CaCl2 → Ca(NO3)2 + 2AgCl
C2H6O + 3O2 → 2CO2 + 3H2O
Explanation:
You see, when we set out to balance chemical reaction equations, ultimately, our aim is to ensure that the number of atoms of each element on the reactant side is exactly the same as the number of atoms of the same element on the products side.
We do this by counting the number of atoms required to have a balanced reaction equation and then adding coefficients in order to have a balanced chemical reaction equation.
This is what have been done in balancing the three reaction equations shown in the answer section.
How is a mixture different from a compound?
Your answer:
20
O A mixture has a chemical formula. A compound does not.
A mixture forms with a chemical reaction. A compound does not.
O A compound is easier to separate than a mixture.
Substances in a compound are chemically combined. The substances in a mixture are not.
Answers
Explanation:
A mixture is made of two or more substances that are not chemically combined whereas a compound is made of 2 or more elements that are chemically combined. The elements that make up the compound are combined in fixed ratios.
the proton nmr spectrum of an aromatic compound, c8h8br2, includes two methyl singlets. its proton-decoupled 13c nmr spectrum displays a total of six peaks. of the following compounds, which structure best fits these data?
Answers
The structure that best fits the given data is 1,4-dibromobenzene.
The presence of two methyl singlets in the proton NMR spectrum indicates the presence of two methyl groups in the compound. This suggests the presence of a substituent attached to the benzene ring.
The proton-decoupled 13C NMR spectrum displays six peaks, indicating the presence of six distinct carbon environments. In 1,4-dibromobenzene, there are two carbon atoms attached to the methyl groups, which gives two peaks. The benzene ring itself has four unique carbon environments, each with a different chemical shift, resulting in four additional peaks.
The structure of 1,4-dibromobenzene matches the data because it contains two methyl groups and displays a total of six peaks in the proton-decoupled 13C NMR spectrum, consistent with the given information.
To learn more about NMR spectrum, here
https://brainly.com/question/30465398
#SPJ4

The Lewis dot diagram for an unidentified element is shown below. Which element
could X represent?
Olithium
calcium
O argon
sulfur
*
X*
Answers
The lewis dot diagram for the given element *X* is of Calcium element where two unpaired electrons are present.
Since calcium has an atomic number of 20 and argon, a noble gas, has an atomic number of 18, calcium is included in the second column of the periodic table.
Since we are discussing the 2⁺ cation, it has already experienced two electron losses. We can tell because every electron has a charge of 1, therefore losing a charge of 1 is equivalent to acquiring a charge of 1.
Additionally, neutral Ca initially had two electrons because it is on the second column or group. Ca₂⁺ has no valence electrons since 2 -2=0.
Drawing the Lewis structure is therefore not too difficult; all you need to do is write Ca and note that it has a 2+ charge.
So, the given element is Calcium.
To know more about lewis dot structure, please refer:
https://brainly.com/question/20300458
#SPJ1
what is the ph of a 1.0 x 10–2-molar solution of hcn? (for hcn, ka = 4.0 x 10–10.) (a) 10 (b) between 7 and 10 (c) 7 (d) between 4 and 7 (e) 4
Answers
The pH of 1 X 10-2 M HCN acid is 5.7
D) between 4 and 7
HCN is weak acid dissociate as
HCN + H2O \rightleftharpoons H3O+ + CN-
Ka = [CN- ][H3O+] / [HCN]
but [CN- ] = [H3O+] = x
Ka = [x][x] / [HCN]
Substitute the value in equation
4.0X 10-10 = [x]2/ 1 X 10-2
[x]2 = 4.0X 10-10 X1 X 10-2 = 4.0 X 10-12
[x] = 2.0 X 10-6 M
Concentration of H3O+ = 2 X 10-6 M
pH = - log[H3O+]
pH = - log (2 X 10-6)
pH = 5.7
What is HCN?Prussic acid, also known as hydrogen cyanide, is a chemical substance with the formula HCN and the structural formula HCN. It is a colourless, incredibly poisonous, and flammable liquid that boils at 25.6 °C (78.1 °F), just slightly above room temperature.
Industrial-scale HCN production makes it a highly prized precursor to a wide range of chemical compounds, from pharmaceuticals to polymers. Production of potassium cyanide and adiponitrile, which are used in mining and plastics, respectively, has large-scale applications. Due to its volatile nature, it is more toxic than cyanide compounds that are solid.
Learn more about Hydrogen cyanide
https://brainly.com/question/27179806
#SPJ1
HELPPPPPP
Algae lives on the back of a spider crab. The crab blends with the shallow water of the ocean floor because of the greenish-brown color of the algae. The algae get a place to live and the crab gets camouflage. What is the name of the relationship that exists between the algae and the spider crab?
Commensalism
Competition
Mutualism
Parasitism
Answers
Answer:
it's a symbiotic mutual relationship
Explanation:
Greenish-brown algae lives on the crab's back, helping the crabs blend in with their enviroment, which makes them less noticeable to predators. the algae provided with a good place to live while the crab receives camouflage.
Answer:
Mutualism
Explanation:
Mutualism; One organism live in, on, or near another, and both gain something they need for survival. Both organisms are heled.