Answers
The color change exhibited by phenolphthalein during a titration of aqueous acetic acid with aqueous sodium hydroxide is pink to colorless.
An indicator is an organic dye which changes color in acidic, basic and neutral medium.
Phenolphthalein is an example of an indicator. When aqueous acetic acid is titrated against aqueous sodium hydroxide, the indicator may be added to the basic solution.
Phenolphthalein has a pink color in a basic medium. As the acid is added gradually, the solution turns colorless at end point.
Hence, the color change exhibited by phenolphthalein during a titration of aqueous acetic acid with aqueous sodium hydroxide is pink to colorless.
Learn more: https://brainly.com/question/2728613
Related Questions
Please help! How many orbits will the Bohr models in the fourth period have?
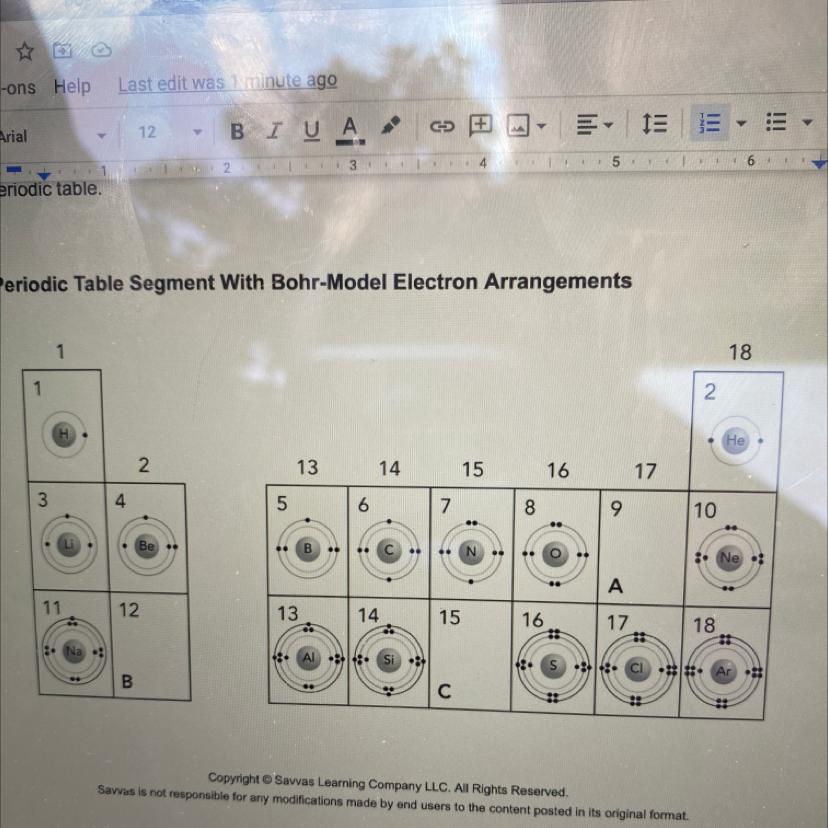
Answers
Answer:
32 electron
Thus,the fourth level can hold up to 32 electrons,2 in the s orbital,6 in the three p orbitals,10 in the five d orbitals,and 14 in the seven f orbitals.
Assuming 100% dissociation, calculate the freezing point ( f ) and boiling point ( b ) of 3.40 AgNO3(aq)
Answers
The freezing point of the silver nitrate solution is - 12.6 degrees Celsius.
What is the freezing point of the solution?We know that freezing point is a colligative property. This implies that it depends on the amount of the substance that is present . We should also know that when we dissolve the silver nitrate in water, the particles of the substance would decrease the freezing point of the water.
Freezing point of water = 0 degrees Celsius
Molality of the solution = 3.40 m
Freezing constant of water = 1.86∘C/m
We now have;
ΔT = K m i
ΔT = 1.86∘C/m * 3.40 m * 2
ΔT = 12.6 degrees
Temperature of the solution = 0 - 12.6 = - 12.6 degrees Celsius
Learn more about freezing point:https://brainly.com/question/3121416
#SPJ1
Use the Debye–Hückel equation to calculate the activity coefficient (
Answers
Answer:
log = ( - 0.51 z 2) / ( 1 + ( / 305)
Explanation:
Use this condensed chemical structure
CH3—CH2—NH2
The condensed chemical structure of ethanamine.
Some facts about the ethanamine molecule:
number of carbon-carbon single (C - C) bonds:
number of carbon-hydrogen single (C H) bonds:
number of nitrogen-hydrogen sing le (N H) bonds:
number of lone pairs:
Answers
Answer:
number of carbon-carbon single (C - C) bonds: 1
number of carbon-hydrogen single (C H) bonds: 5
number of nitrogen-hydrogen sing le (N H) bonds:2
number of lone pairs: 1
Explanation:
Ethanamine is a colourless gas having a strong 'ammonia- like' odour. It contains the -NH2 group which makes it an amine. It contains one carbon-carbon bond, five carbon-hydrogen bonds and two nitrogen-hydrogen bonds.
Nitrogen, being sp3 hybridized in the compound has a lone pair of electrons localized on one of the sp3 hybridized orbitals of nitrogen while one sp3 hybridized orbital of nitrogen is used to form a carbon-nitrogen bond. The other two sp3 hybridized orbitals on nitrogen are used to form the two nitrogen-hydrogen bonds.
What is the element of lowest atomic number whose electronic configuration has four completely filled p sub shells
Answers
The element of lowest atomic number whose electron configuration has 4 completely filled p orbitals is, Xenon, Xe with electron configuration:1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶.
According to the question, we are required to identify the element with the lowest atomic number whose electronic configuration has four completely filled p sub-shells.
Evidently, the shell with energy level one has no p orbital.
Therefore, the existence of p orbitals in electron configuration begins with shell energy level, n=2.However, we must know that the p-orbital comprises of 3 sub-orbitals namely;p(x) , p(y) and p(z) with two electrons of opposing spin in each sub orbital.
Therefore, the total number of electrons in the p orbital is 6 electrons.In essence, the element in question should have electron configuration;
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶.The electron configuration above contains 54 electrons and has atomic number, 54.
In essence, the element of lowest atomic number whose electron configuration has 4 completely filled p orbitals is, Xenon, Xe.
Read more:
https://brainly.com/question/2183111
The image shows a dam. Which descriptions best fit the labels? O X: kinetic energy Y: potential energy O X: potential energy Y: kinetic energy O X: mechanical energy Y: electrical energy O X: electrical energy Y: mechanical energy .Y . please help i will mark you brainlist

Answers
Answer:
b
Explanation:
Answer:
X: mechanical energy
Y: electrical energy( C)
Explanation:
True or False: Air can be made to vibrate.
Answers
Answer
False
Explanation:
Calculate the mass percent of each component in the following solution.
159 g NiCl2 in 500 g water
% Nicla
% water
Answers
Answer:
% NiCl2 = 24.13%
% water = 78.57%
Explanation:
Mass percentage = mass of solute/mass of solution × 100
According to this question, a solution contains 159 g of NiCl2 in 500 g of water. Hence, mass of the solution is calculated as follows:
Mass of solution = 159g + 500g
Mass of solution = 659g
Therefore;
A) % Mass of NiCl2 in solution = mass of NiCl2/mass of solution × 100
% Mass of NiCl2 in solution = 159/659 × 100
% Mass of NiCl2 in solution = 0.2413 × 100
= 24.13%
B) % Mass of water in solution = mass of water/mass of solution × 100
% Mass of water in solution = 500/659 × 100
% Mass of water in solution = 0.7587 × 100
% Mass of water in solution = 75.87%
You have a solid object of unknown composition and mass. You determined that when this object absorbed 1.000 X 10^2J, its temperature increased by 2.0K. Calculate the objects heat capacity
Answers
Answer:
100 rbed KJ |0| +2k
Explanation:
The number of electrons in 1.6 gram CH, is:
Answers
Answer:
6.022 x 10²³ electrons
Explanation:
How was the periodic law rearranged to become the periodic table, and what is its purpose?
Answers
Answer:
At earlier times elements were arrangrd on the basis of their atomic masses to study their properties easily but there were various problems like isotopes needs different position in perodic table.
After discovery of atomic number modern periodic table was created on the basis of atomic number (number of proton) that is unique for each element and varies regularly. So study of a group of elements is much easier than each element seperately that is why modern periodic table was formed.
Explanation:
Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3g/cm3, which translates to an increase in pressure of 1.00 atmatm for every 10.0 mm of depth below the surface. Therefore, for example, at 10.0 mm, the net pressure is 2.00 atmatm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface
Answers
Answer:
The ration of molar concentration is "2.5".
Explanation:
The given values are:
Average density of salt water,
= \(1.03 \ g/cm^3\)
Net pressure,
= \(2.00 \ atm\)
Increase in pressure,
= \(1.00 \ atm\)
Now,
The under water pressure will be:
= \(\frac{15 \ m}{10}\times 1 \ atm +1 \ atm\)
= \(1.5\times 1+1\)
= \(1.5+1\)
= \(2.5 \ atm\)
hence,
The ratio will be:
= \(\frac{(\frac{n}{V})_{15m} }{(\frac{n}{V})_{surface} }\)
or,
= \(\frac{P}{P_s}\)
= \(\frac{2.5}{1}\)
= \(2.5\)
If I formed an ion, what would it's charge likely be and why?
A. -2 because it gains 2 electrons
B. +2 because it gains 2 electrons
C. +4 because it loses 4 electrons
D. -6 because it gains 2 electrons
Answers
Answer: -2 because it gains 2 electrons
Explanation:
An ion is considered to be an atom which is formed by gain or loss of electrons.
The ions are classified into two which are called the cation and anion.
The cation is classified as the positive charge formed by loss of electrons.
The anion is classified as the negative charge ion formed by gain of electrons.
On gaining two electrons, the atom would occupy -2 charge.
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
24. which is the correct iupac name for the following

Answers
24. The IUPAC name of the compound is 2,4-dimethyl-1-pentanal (Option B)
25. The IUPAC name of the compound is 5-methyl-3-octanone (Option C)
How do I obtain the IUPAC name?The international union of pure and applied chemistry (IUPAC) standard for naming organic compounds is given below:
Locate the longest continuous carbon chain.Identify the functional group. Identify the substituent groups attached to the compound.Give the substituents groups the lowest count by considering the functional group.Combine the above to get the name of the compound.24. The name of the compound is given as follow:
The longest continuous carbon chain is 5 i.e pentaneThe functional group is alkanal (aldehyde). Thus, the parent name will be pentanalThe substituent group attached is methylThere are two methyl groups located at carbon 2, and 4Thus, the IUPAC name of the compound is:
2,4-dimethyl-1-pentanal (Option B)
25. The name of the compound is given as follow:
The longest continuous carbon chain is 8 i.e octaneThe functional group is alkanone (ketone). Thus, the parent name will be octanoneThe substituent group attached is methyl and it is located at carbon 5Thus, the IUPAC name of the compound is:
5-methyl-3-octanone (Option C)
Learn more about IUPAC naming:
https://brainly.com/question/16180944
#SPJ1
How many grams are in 0.040 moles zirconium (Zr)?
Answers
Answer:
91.224 gram
Explanation:
Answer:
3.64896 grams Zr
Explanation:
Given: amount of moles of Zr: 0.040
Find: the amount of grams of Zr
1. Find molar mass of Zr
- after looking at the periodic table, you can tell it's 91.224 g/mol
2. Solve
(\(\frac{0.040 mol Zr}{1}\))( \(\frac{91.224 g Zr}{1 mol Zr}\))
- the mol units get cancelled out, and you're left with 0.040 x 91.224 g
3. Multiply that out: 3.64896 grams of zirconium
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
iron (ll) is available to bond with Chloride ion. How many of each type of ion will bond to form an ionic compound?
A) 2 iron , 3 chloride
B) 3 iron , 1 chloride
C) 1 iron , 2 chloride
D) 2 iron, 1 chloride
Answers
,,
Pay attention to charges and roman numerals
Water can be an atom.
True
False
Answers
true because I asked my sister
Answer:
false
Explanation:
yep
37.4 g of aluminum chloride reacts with bromine gas to yield aluminum bromide and chlorine gas. How many liters of bromine gas is needed to completely react with aluminum chloride? Assume both gases are at STP.
Answers
Answer:
9.43 L Br₂ (g)
Explanation:
First, write a balanced reaction equation. Then use dimensional analysis to convert from grams of aluminum chloride to moles of aluminum chloride (with molar mass from the periodic table), from moles of aluminum chloride to moles of bromine gas (using the balanced equation), and from moles of bromine gas to liters of bromine gas (remember at STP, 1 mol = 22.4 L). See the attached image for the work out. Finally, account for sig figs to get 9.43 L of bromine gas

Which of the following is NOT a natural resource? Check all that apply.
1. Air
2. Water
3. Plastic
4. Sunlight
5. Cotton
6. Glass
7. Coal
8. Copper
Answers
While being made from natural resources, electricity does not constitute a natural resource because it goes through several procedures to produce it.
What are the seven categories of natural resources?Oil, coke, nat gas, metals, stone, or sand are examples of natural resources. Other natural resources include water, soil, sunlight, air, and so on. Natural resources have value because they enable life and provide for human needs.
A natural resource is land?Financially referred to it as land and raw materials, land resources (also known as natural resources) are found naturally within ecosystems that are mostly unaltered by civilization. A natural resource's biodiversity levels across different habitats are frequently used to describe it.
To know more about several visit:
https://brainly.com/question/13758372
#SPJ1
5. What is the amount of moles of nitrous oxide gas (N2O) used to sedate Mr. Muthbefore his wisdom teeth are pulled? The pressure is 2.5 atm, a temperature of 25.0°C,and volume of 1.67L?
Answers
0.171moles
Explanations
According to the ideal gas equation;
\(PV=nRT\)where:
• P is the, pressure ,in atm
,• V is the ,volume ,in litres
,• n is the ,number of moles ,of gas
,• R is the, boltzmann constant
,• T is the, temperature ,in Kelvin
Given the following parameters
P = 2.5atm
V = 1.67L
T = 25.0°C = 25 + 273 = 298K
R = 0.08205Latm/molK
Substitute the given parameters into the formula
\(\begin{gathered} n=\frac{PV}{RT} \\ n=\frac{2.5\times1.67}{0.08205\times298} \\ n=\frac{4.175}{24.4509} \\ n=0.171mole \end{gathered}\)Hence the amount of moles of nitrous oxide gas used is 0.171moles
Convert this measurement, 3.2x10-3 mm, from scientific notation to standard notation
Answers
Answer:
0.0032mm
Explanation:
Given dimension:
Measurement = 3.2 x 10⁻³mm
Unknown:
Write in standard notation from scientific notation = ?
Solution:
The standard notation is the way we normally write.
For example; standard notation of 1 million is 1000000
Now;
3.2 x 10⁻³ = \(\frac{3.2}{1000}\) = 0.0032mm
3.2x10⁻³ mm in standard notation is 0.0032 mm. The normal or generally accepted manner of representing or expressing anything is referred to as standard notation.
Standard notation refers to the conventional or widely accepted way of representing or expressing something. In mathematics, for example, standard notation refers to the commonly used symbols and formats for writing mathematical expressions, equations, or numbers. The use of standard notation ensures uniformity, ease of understanding, and communication among mathematicians and students. Powers of 10 are used in scientific notation to express extremely large or extremely small quantities. 3.2x10⁻³ mm in standard notation is 0.0032 mm.
To know more about standard notation, here:
https://brainly.com/question/29069315
#SPJ6
Put these in order from least amount of inertia to greatest amount of inertia.
A Toy Car
A Bicycle
A jet airplane
A Truck
Answers
1) Toy car
2) Bicycle
3) truck
4) Jet Airplane
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins.
Dispersion Forces; Dipole-Dipole Forces; Hydrogen Bonding Forces
Kr, H2O, CHCI3, HF, C2H6, HBr
Answers
Answer:
Kr- Dispersion Forces
H2O- Hydrogen Bonding
CHCI3- Dipole-Dipole Forces
HF- Hydrogen Bonding
C2H6- Dispersion Forces
HBr- Hydrogen Bonding Forces
Explanation:
Dispersion forces occurs in all substances. They are the dominant intermolecular interaction in all non polar substances such as C2H6 and Kr.
Hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom such as Cl, Br, O etc. It is the dominant intermolecular interaction in HF, HBr and H2O.
Dipole-Dipole interactions occur when a permanent dipole exists in a molecule such as in CHCI3
What is the relationship between temperature and kinetic energy?
Answers
Answer:
"[Temperature is a measurement of the average kinetic energy of the molecules in an object or a system. Kinetic energy is the energy that an object has because of its motion. The molecules in a substance have a range of kinetic energies because they don't all move at the same speed.]"
Answer:
Temperature is directly proportional to the average translational kinetic energy of molecules in an ideal gas
Explanation:
Which equations are balanced?
* Na₂O + H₂O → 2NaOH
* CaO + H₂0 ➜ Ca(OH)2
* 02K + Cl₂ ⇒ 2KCI
* Al + HCl → AlCl3 + H₂
Answers
Answer:
Na₂O + H₂O → 2NaOHCaO + H₂O ➜ Ca(OH)22K + Cl₂ ⇒ 2KCIFor this assignment, you will be creating your own potential energy diagrams for three chemical reactions. Then you will use your diagrams to find the value of △H for each reaction.
Answers
By analyzing the potential energy diagrams and calculating ΔH using bond energies, we can determine whether a reaction is exothermic (ΔH < 0) or endothermic (ΔH > 0).
To create potential energy diagrams for chemical reactions and determine the value of ΔH (the change in enthalpy) for each reaction, we need to understand the basic concepts and steps involved.
Potential Energy Diagram: A potential energy diagram is a graphical representation of the energy changes that occur during a chemical reaction. The vertical axis represents the potential energy, while the horizontal axis represents the progress of the reaction.
Reactants and Products: Identify the reactants and products involved in each reaction. Assign them appropriate labels on the potential energy diagram.
Activation Energy: Determine the activation energy (Ea) for each reaction. It represents the energy barrier that must be overcome for the reaction to occur. On the diagram, the reactants' energy level is typically higher than the products' energy level, with the activation energy peak in between.
Transition State: Locate the highest point on the potential energy diagram, which represents the transition state or activated complex. This point indicates the highest energy level during the reaction.
ΔH Determination: ΔH represents the difference in enthalpy between the reactants and products. It can be determined by examining the vertical distance between the reactants' energy level and the products' energy level on the potential energy diagram.
ΔH Calculation: ΔH can be calculated using the formula ΔH = Σ (bond energies of reactants) - Σ (bond energies of products). The bond energies are the energy required to break a particular bond or released when a bond is formed.
For more such question on energy. visit :
https://brainly.com/question/28109294
#SPJ8
What volume of concentrated 15M H2SO4 is required to prepare 0.75 liters of a 6.0M solution?
Answers
Answer:
30 ml
Explanation:
share the imporatant lesson that you have learned in organic chem
Answers
We study the reactions that chemists utilise to create bizarre carbon-based structures in organic chemistry.
The study of the makeup, properties, and responses of organic compounds including organic materials, or matter in any of its many forms that contains carbon atoms, is the subject of the branch of science known as organic chemistry. Their structural formula is determined by study of structure.
We will study the reactions that chemists utilise to create bizarre carbon-based structures in organic chemistry, in addition to the analytical techniques used to characterise them. We'll also consider the molecular reaction mechanisms that are driving those reactions.
To know more about organic chemistry, here:
https://brainly.com/question/14623424
#SPJ1