Two samples are poured through filters. Sample #1 is separated into two substances by the filtration, while Sample #2 remains unchanged by filtration.
Which of the following is the most reasonable conclusion based on these observations?
Answers
Answer: That sample 2 is a different substance because it is unfiltered and combined
Explanation:
Sample #1 is a heterogeneous mixture and Sample #2 is either a homogeneous mixture or a pure substance, based on their reactions to filtration.
Explanation:The observation from the filtration experiment of the two samples suggests that Sample #1 is a heterogeneous mixture while Sample #2 is a pure substance or a homogeneous mixture. The filtration process is capable of separating the components of a heterogenous mixture like sand and water, but not the components of a homogenous mixture or pure substance, such as salt water or pure water.
Learn more about Filtration of Mixtures here:https://brainly.com/question/33821622
#SPJ2
Related Questions
¿Cómo se llama el grupo IIA de la tabla periódica?
Answers
Answer:
los metales alcalinotérreos: berilio (Be), magnesio (Mg), calcio (Ca), estroncio (Sr), bario (Ba) y radio (Ra).
o simplemente llamado grupo 2A
Explanation:
20. What are the metals in the center of the periodic table referred to as?
Answers
The metals in the center of the periodic table are called transition metals. There are 38 transition metals in total.
Answer:
They are called transition metals.
An electron in the n = 6 level emits a photon with a wavelength of 410. 2 nm. To what energy level does the electron move?.
Answers
The electron will move to 2nd energy level as n = 2
What are energy levels?An energy level (also called an electron shell) is a fixed distance from the nucleus at which an electron can exist. Electrons are small, negatively charged particles in atoms that move around a central, positive nucleus. Energy levels are a bit like stair steps. The energy emitted at a particular transition is equal to the energy difference between the two energy levels.
E(n) = −1/n² × 13.6eV
For the given case,
λ = 410.2 nm
h = 6.62 × 10⁻³⁴ j h⁻¹
c = 3 × 10⁸
E = hc/λ
E = 6.62 × 10⁻³⁴× 3 × 10⁸/410.2 × 10⁻⁹
E = 0.048 × 10⁻¹⁷ joules
E = 2.99 eV
E = -13.6 1/n²
Since, n = 6
E = -13.6 1/6²
E = -0.38 eV
ΔE = E₂ - E₁
2.99 = -0.38 - E
-3.37 = E
-3.37 = -13.6 1/n²
n = 2
To know more about energy levels visit:
https://brainly.com/question/17396431
#SPJ4
2. A gas container is initially at 40 mm Hg and 77K (liquid nitrogentemperature.) What will the pressure be when the container warmsup to room temperature of 298K?
Answers
ANSWER
The final pressure of the gas is 155mmHg
EXPLANATION
Given that:
The initial pressure of the gas is 40mmHg
The initial temperature of the gas is 77K
The final temperature of the gas is 298K
To find the final pressure, follow the steps below
In the given data, the volume of the container is fixed. Hence, the process is an Isochoric.
Step1: Write the gas law at constant volume
\(\frac{P1}{T1}=\frac{P2}{T2}\)Step 2: Substitute the given data into the formula in step 1 to find P2
\(\begin{gathered} \frac{40}{77}=\frac{P2}{298} \\ \text{ Cross multiply} \\ \text{ 40}\times\text{ 298 }=\text{ 77}\times P2 \\ 11920\text{ }=\text{ 77}\times\text{ P2} \\ \text{ Divide both sides by P2} \\ \text{ P2 }=\frac{11920}{77} \\ P2\text{ }=154.8mmHg \\ P2\approx155mmHg \end{gathered}\)Hence, the final pressure of the gas is 155mmHg
What is the amount of heat absorbed when the temperature of 75 grams of water increases from 20 to 35 Celsius
Answers
The heat that is absorbed by the object is 4.7 kJ.
What is the amount of heat?We have to know that heat is absorbed or evolved when we heat an object up or when we cool it down. In this case, we have been told that 75 grams of water increases from 20 to 35 Celsius.
We ought to know that the specific heat capacity of water is about 4.2 J/g/°C and this information is very important.
Then we have;
H = mcdT
m = mass of the water
c = specific heat capacity
dT = temperature change
Thus;
H = 75 * 4.2 * (35 - 20)
H = 4.7 kJ
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
what does a lineweaver-Burk plot look like?
Answers
The lineweaver-Burk plot is applicable for enzyme kinetics and image of same is attached below.
Enzymes are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life.
When used for determining the type of enzyme inhibition, the Lineweaver–Burk plot can between distinguish competitive, pure non-competitive and uncompetitive inhibitors. The various modes of inhibition can be compared to the uninhibited reaction.
Learn more about enzymes,here:
https://brainly.com/question/31385011
#SPJ4

which artwork was created through the use of ceramics or the medium of pottery? which artwork was created through the use of ceramics or the medium of pottery?
Answers
There are countless artworks that have been created through the use of ceramics or the medium of pottery. Ceramic art is an ancient art form that has been used for practical and artistic purposes for thousands of years.
Pottery is a type of ceramic art that involves molding clay into various shapes and firing it at high temperatures to create a durable and functional object.
Some examples of artwork that have been created through the use of ceramics or pottery include vases, bowls, plates, sculptures, and even tiles and mosaics. These objects can be decorated with intricate patterns, glazes, and other embellishments that add to their aesthetic value.
Ceramic art has been an important part of many cultures throughout history, including ancient China, Greece, and the Americas. Today, ceramic artists continue to create beautiful and unique works of art using this versatile medium.
In summary, there are countless artworks that have been created through the use of ceramics or the medium of pottery. These objects can be both functional and decorative, and have been an important part of human artistic expression for thousands of years.
To know more about pottery visit:
https://brainly.com/question/32053220
#SPJ11
Ocean currents bring warm from the equator towards earth?
Answers
Answer:Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
Explanation:
Answer:
Explanation:
Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
hope it helps!
Find the pH of a 0.135 M aqueous solution of periodic acid (HIO.), for which K -2.3 x 10-2 Select one: a. 1.25 b. 3.28 c. 1.17 d. 1.34 e. 1.64
Answers
The pH of a 0.135 M aqueous solution of periodic acid (HIO4), for which K = 2.3 x 10^-2, is 1.64
The balanced equation for the dissociation of HIO4 in water is:
HIO4 + H2O ⇌ H3O+ + IO4-
The equilibrium constant expression for this reaction is:
\(K = [H3O+][IO4-]/[HIO4]\)
Given that K = 2.3 x 10^-2 and [HIO4] = 0.135 M, we can solve for [H3O+]:
\(2.3 x 10^-2 = [H3O+][IO4-]/0.135\\[H3O+] = 0.135 x 2.3 x 10^-2 / [IO4-]\)
Now, we need to find [IO4-]. Since the dissociation of HIO4 is a monoprotic acid, the initial concentration of HIO4 is equal to the concentration of IO4- produced:
[IO4-] = 0.135 M
Substituting this value into the equation for [H3O+], we get:
[H3O+] = 0.135 x 2.3 x 10^-2 / 0.135
[H3O+] = 2.3 x 10^-2
Finally, we can calculate the pH of the solution using the definition of pH:
pH = -log[H3O+]
pH = -log(2.3 x 10^-2)
pH = 1.64
Learn more about pH : https://brainly.com/question/27858858
#SPJ11
Which pair of properties describes the elements in Group 18?
F. They are chemically stable and liquid at room temperature.
G. They have eight valence electrons and are flammable.
H. They are magnetic and boil at low temperatures.
J. They are gaseous at room temperature and chemically stable
Answers
The correct option is J, They are gaseous at room temperature and chemically stable" which correctly describes the properties of the elements in Group 18.
Room temperature is typically defined as the temperature range at which a substance or reaction is carried out under normal laboratory conditions, without the need for specialized equipment or procedures to control the temperature. Room temperature is usually considered to be around 20-25 degrees Celsius (68-77 degrees Fahrenheit), although this can vary slightly depending on the specific laboratory or experiment.
At room temperature, most common substances are in a stable, solid or liquid state, and many chemical reactions can take place at a reasonable rate without the need for additional heating or cooling. However, it is important to note that certain reactions or materials may require more precise temperature control in order to ensure accurate results or prevent safety hazards.
To learn more about Room temperature visit here:
brainly.com/question/8739174
#SPJ4
2h2 + 02 = 2h20
what is the volume of steam could be produced at stp if 12.8 g of oxygen reacts with excess hydrogen
Answers
The volume of steam that could be produced at STP if 12.8 g of oxygen reacts with excess hydrogen is 17.92 L
How to determine the volumeWe'll begin by obtaining the mole of steam produced. This can be obtained as follow:
Mass of O₂ = 12.8 gMolar mass of O₂ = 2 × 16 = 32 g/molMole of O₂ = 12.8 / 32 = 0.4 mole2H₂ + O₂ -> 2H₂O
From the balanced equation above,
1 mole O₂ reacted to produce 2 moles of steam, H₂O
Therefore,
0.4 mole O₂ will react to produce = 0.4 × 2 = 0.8 mole of steam, H₂O
Finally, we shall determin the volume as follow:
At standard temperature and pressure (STP),
1 mole of H₂O = 22.4 L
Therefore,
0.8 moles of H₂O = (0.8 mole × 22.4 L) / 1 mole
0.8 moles of H₂O = 17.92 L
Thus, we can conclude that the volume produced is 17.92 L
Learn more about volumes at STP:
https://brainly.com/question/22311771
#SPJ1
can someone suggest me topics for chemistry project work?
Answers
Answer:
Which
PLEASE MARK AS BRAINLIEST ANSWER
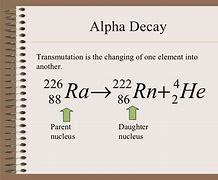
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
What is the mass number of an element that has 8 protons, 9 neutrons and
7 electrons?
Answers
Answer:
17
Explanation:
mass number = # of protons + # of neutrons
PLEASE HELP!! i am very stuck

Answers
Answer:
Explanation:
Molar mass of Ca(Cn)2 = 92. 11 mol/g
If you had 5.0 g of material that needed to be purified, would you opt for using TLC or column chromatography to purify your material? Explain your answer.
Answers
If you have 5.0 g of material that needs to be purified, I would recommend using column chromatography to purify your material.
Column chromatography is more suitable for larger quantities and can separate complex mixtures more efficiently than TLC (thin-layer chromatography), which is typically used for smaller-scale analysis and preliminary identification of components.
It is a precursory method for purifying substances based on how hydrophobic or polar they are. The molecular mixture in this chromatography procedure is divided based on how differently it partitions between a stationary phase and a mobile phase.
The compound mixture is transported by a mobile phase through a stationary phase in a separation that is comparable to that of TLC.
Elution is a chromatographic process that involves utilising a solvent to remove an adsorbate from a solid adsorbing substrate.
To know more about column chromatography click here:
https://brainly.com/question/30296545
#SPJ11
What is occurring with the energy and molecules in a metal that is melting?
Answers
Answer:
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point.
identify reagents that can be used to convert 1-pentyne into 1-bromopentane.select answer from the options below1) h2, lindlar's catalyst; 2) hbr (1 equiv.), roor1) hbr (1 equiv.), roor; 2) h2, lindlar's catalyst1) hbr (1 equiv.); 2) h2, pd1) hbr (1 equiv.); 2) h2, lindlar's catalyst1) h2, lindlar's catalyst; 2) hcl (1 equiv.)
Answers
Reagents that can be used to convert 1-pentyne into 1-bromopentane is HBr (1 equiv.), ROOR.
The reaction of 1-pentyne with HBr (hydrogen bromide) in the presence of a radical initiator such as ROOR (e.g., benzoyl peroxide) will produce 1-bromopentane.
This is a radical addition reaction where the H-Br bond is cleaved homolytically to form Br radical, which attacks the alkyne to form a more stable radical.
The radical then combines with another H-Br molecule to form the product 1-bromopentane.
To know more about Reagents visit:
brainly.com/question/31228572
A student adds alka-seltzer to pure water and the final ph of the solution is 8.3. what ion was released by alka-seltzer to change the ph?
Answers
The ion released by alka-seltzer to change the pH of the solution is the hydroxide ion (OH-). When alka-seltzer is added to water, it undergoes a chemical reaction that releases hydroxide ions.
These hydroxide ions then react with the water molecules, resulting in an increase in the concentration of hydroxide ions in the solution. This increase in hydroxide ion concentration leads to an increase in pH, making the solution more basic. The pH scale ranges from 0 to 14, with a pH of 7 considered neutral, values below 7 acidic, and values above 7 basic.
Since the final pH of the solution after adding alka-seltzer is 8.3, it indicates that the solution has become more basic due to the release of hydroxide ions. In summary, when alka-seltzer is added to water, it releases hydroxide ions, which react with water to increase the concentration of hydroxide ions and raise the pH of the solution. When alka-seltzer is added to pure water, it dissolves and undergoes a chemical reaction. This reaction results in the release of hydroxide ions (OH-) into the solution.
To know more about solution visit:
https://brainly.com/question/1616939
#SPJ11
at 35 °c, the ph of 0.10 m naoh is 12.68. what is the value of kw at this temperature?
Answers
At 35°C, the value of Kw (the ionic product of water) is 1.16 x 10^-14.
The pH of a solution is determined by the concentration of hydrogen ions (H+) in the solution. The pH of a 0.10 M NaOH solution at 35°C is 12.68. The pH of a solution is related to the ionic product of water (Kw), which is defined as the product of the concentrations of the hydrogen (H+) and hydroxide (OH-) ions in the solution. At 35°C, the value of Kw is 1.16 x 10^-14.
At 35°C, the value of Kw (the ionic product of water) is 1.16 x 10^-14.
learn more about ionic here
https://brainly.com/question/22435035
#SPJ4
Balance this reaction.
__CO +__ H₂ —> __CH3OH
options for blanks: (blank,2,3,4)

Answers
Answer:
1CO + 2H₂ —> 1CH3OH
Explanation:
When balancing any chemical equation given, you identify the amount of reactants and amount of products.
From our given equation;
__CO +__ H₂ —> __CH3OH
Reactants
C = 1
O = 1
H = 2
Products
C = 1
O = 1
H = 3 + 1 = 4
This shows that Hydrogen is not balanced we require 2 to balance it. Thus giving us;
1CO + 2H₂ —> 1CH3OH,
Which is balanced.
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
1. Which combinations of substances resulted in a chemical change?
I'm honestly so confused by this entire lab, any help would be appreciated. If you're doing k12 chem, reaction of metals lab.
2. For each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table.
3. Were there any metallic compounds that did not react with either the acid or the base? Write the type of metal, based on your examination of the periodic table.
4. Make a general statement about the reactivity of the metals in this experiment.

Answers
Answer:
. For each metal that participated in a chemical change, write the type of metal it is, based on yourexamination of the periodic table.Answer: Ammonium Vanadate appears to be mostly a post transition metal. Manganese sulfate also hasmore post transition atoms than transition atoms. Iron nitrate is a transition metal. The cobalt nitrate is atransition metal. Copper nitrate is a transition metal. Zinc nitrate is also a transition metal.(5 points)3. Were there any metallic compounds that did not react with either the acid or the base? Write the type ofmetal, based on your examination of the periodic table.4. Make a general statement about the reactivity of the metals in this experiment.Answer: The transition metals in this experiment were all reactive when mixed with one of the twosubstances. The alkali and alkaline earth metals, however, did not react at all.
Explanation:
Are all the atoms in the same plane for cyclohexanone.
Answers
Answer:
the atoms in cyclohexanone are not in the same
Explanation:
but they are in a benzene ring! Cyclohexane has only sigma bonded carbons with bonds that stick out of the plane
An atom has 17 protons and 18 neutrons.
Which symbol represents that atom, including its mass number?
Answers
Answer:
Cl
Explanation:
cl has mass number 35 and its atomic number is 17 so number of proton =atomic number 17 and number of neutron is =mass number -number of proton =35-17 =18
Give two differences between the physical properties of the elements in Group 1 and those of the transition elements. [2 marks
Answers
Due to their stronger metallic bonding and more compact atomic structure, the transition elements have higher melting and boiling temperatures and are often denser than the alkali metals.
What are group one metals' two physical characteristics?Elements from Group 1 have similar properties. All of them are supple silver metals. These metals are extremely reactive and have low melting temperatures due to their low ionisation energy. As you descend the chart, this family becomes more reactive.
What are the transitional elements?The d orbitals of transitional elements are only partially filled. A transition element is defined by IUPAC as an element that may form stable cations and has an electron d subshell that is only partly filled.
To know more about transition elements visit:-
https://brainly.com/question/1948991
#SPJ1
what is the heat, q , in joules transferred by a chemical reaction to the reservoir of a calorimeter containing 95.0 g of dilute aqueous solution ( c
Answers
Heat is the result of the movement of kinetic energy within a material or an item, or by a source of energy to a material or an object.
What in chemistry is a heat?Energy that is transported from a region of greater temperature to one of lower temperature is known as heat.Joule is the SI unit (J).Heat is another factor that causes the phase transition.
What does fundamental science mean by heat?heat is the energy that moves through one body to the other when temperatures are different.Heat passes from the hotter to the colder body when two bodies with differing temperatures are brought together.
To know more about chemical reaction visit:
https://brainly.com/question/22817140
#SPJ4
21) Technetium-99 is a nuclear isomer that is used in tens of millions of medical diagnostic procedures annually and has a half-life of six hours. Suppose you have a 100mg sample of Technetium-99. a) Write a function that models the sample. b) Approximate how much of the sample will be remaining after one day. 4
Answers
After one day, approximately 8.67mg of the sample will be remaininga) The function that models the sample of Technetium-99 is given by
f(t) = P₀e^(-kt)
Where,P₀ = initial quantity = 100mgk = decay constantt = timef(t) = remaining quantity after t time.
A half-life of 6 hours is given. The decay constant can be found using the half-life formula:
T½ = (ln 2)/k6
= (ln 2)/kk
= (ln 2)/6f(t)
= P₀e^(-kt)f(t)
= 100e^(-0.1155t)mg
b) After one day, 24 hours = 4 half-lives Remaining amount,
f(t) = P₀e^(-kt)f(24)
= 100e^(-0.1155 × 24)
= 100e^(-2.772)
≈ 8.67mg
After one day, approximately 8.67mg of the sample will be remaining. The function that models the sample is
f(t) = 100e^(-0.1155t), where t is time in hours and f(t) is the remaining quantity in milligrams.
To know more about Technetium-99 visit:-
https://brainly.com/question/29970596
#SPJ11
After one day, approximately 8.67mg of the sample will be remaininga) The function that models the sample of Technetium-99 is given by
f(t) = P₀e^(-kt)
Where,P₀ = initial quantity = 100mgk = decay constantt = timef(t) = remaining quantity after t time.
A half-life of 6 hours is given. The decay constant can be found using the half-life formula:
T½ = (ln 2)/k6
= (ln 2)/kk
= (ln 2)/6f(t)
= P₀e^(-kt)f(t)
= 100e^(-0.1155t)mg
b) After one day, 24 hours = 4 half-lives Remaining amount,
f(t) = P₀e^(-kt)f(24)
= 100e^(-0.1155 × 24)
= 100e^(-2.772)
≈ 8.67mg
After one day, approximately 8.67mg of the sample will be remaining. The function that models the sample is
f(t) = 100e^(-0.1155t), where t is time in hours and f(t) is the remaining quantity in milligrams.
To know more about Technetium-99 visit:-
https://brainly.com/question/29970596
#SPJ11
what is potential energy
Answers
Answer:
The energy possessed by a body by virtue of its position relative to others, stresses within itself, electric charge, and other factors.
so simple question.
Answer:
Potential energy is stored energy that an object has because of its position.
Explanation:
Consider an example where a ball is thrown from a tall building. The point at which the ball is at its maximum height is said to have the maximum potential energy. As the ball gradually falls down, the potential energy decreases and turns into another form of energy, kinetic energy (energy in the form of motion). The ball at its peak position had stored energy, however, as soon as it begins moving, the stored energy will become less.
You can think of it this way where the ball has less and less distance to cover when falling downwards from the building. This shorter distance covered by the ball is proportional to the potential energy, so when the ball is about to hit the ground, the potential energy will be close to 0.
I hope this makes more sense!
When you put a liquid, some pour Out of a container really quick, like soda pop. Others, pour out really slow like syrup and honey. What is this property of a liquid called?
A.liquidity
B.melting point
C.surface tension
D.viscosity
I’ll mark you brainliest
Answers
Answer:
Viscosity because the rest are different